Content Review:
Textbook: Chapter 10
Links: Understanding Chemistry Virtual Textbook
Agenda:
1. Chemistry of alkanes and alkenes
2. Chemistry of alcohols, halogenoalkanes and benzene
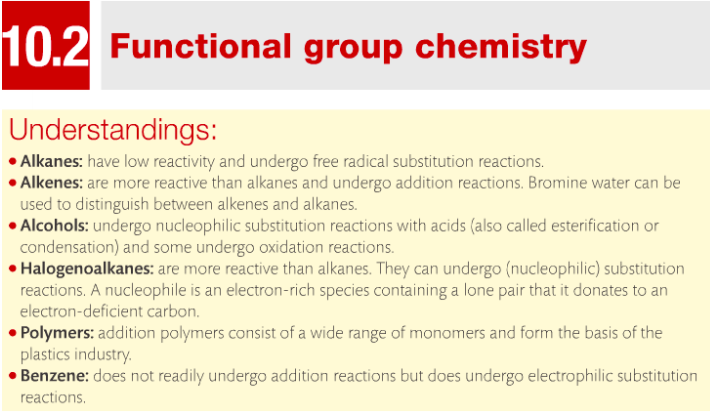
Essential Idea: Describe the reactivity of alkanes, alkenes, alcohols, halogenoalkanes, polymers and benzene.
Combustion: Alkanes as fuels for engines and household heating because they produce significant amounts of energy when burned (think gasoline). Combustion reactions are highly exothermic because of the energy released when bonds are formed in carbon dioxide and water vapor…the products of all combustion reactions.
When oxygen is limited, incomplete combustion occurs, water and carbon monoxide are produced. When oxygen is nearly depleted, carbon will also be produced.
Burning of fossil fuels leads to production of greenhouse gases (carbon dioxide, water vapor) which means they absorb infrared radiation and contribute to global warming and climate change.
Substitution: Halogenation
The main type of reaction alkanes can undergo is substitution. This occurs when a reactant (a halogen) replaces one of the hydrogen atoms in a hydrocarbon. Ultraviolet light is necessary to break the covalent bond in Cl-Cl, which splits it into chlorine atoms that have unpaired electrons. These are called free radicals.
Addition of hydrogen turns alkenes into alkanes.
Addition of halogens produces halogeno- compounds. They happen fast at room temperature and are accompanied by the loss of color of the reacting halogen (p. 331).
Addition of halogen halides (HCl, HBr, HI) react with alkanes to produce halogenoalkanes. Take place rapidly.
Order of reactivity: HI > HBr > HCl due to decreasing bond strength down group 17.
Adding water converts an alkene into an alcohol. Uses sulfuric acid as a catalyst and requires heat with steam. This is an industrial reaction because ethanol is a very important solvent and is manufactured on a large scale.
Polymerization. Alkenes can be joined together to produce long chains called addition polymers (p. 332-333). Polymers can contain thousands of monomers. Repeating units have open bonds on each end (see text).
Alkenes regularly undergo addition reactions and are used as starting materials in the manufacture of many industrial chemicals.
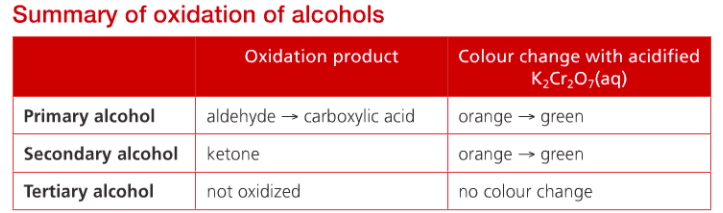
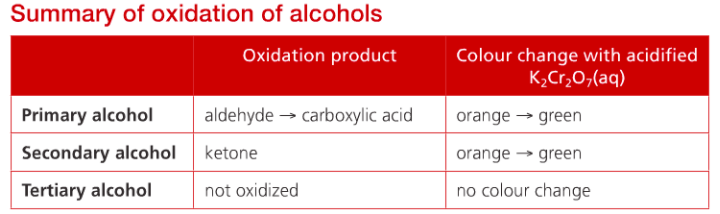
Oxidation of alcohols
Combustion involves the complete oxidation of alcohol molecules, but it is possible for them to react with oxidizing agents that selectively oxidize the carbon atom attached to the alcohol (-OH) group, which keeps the carbon skeleton intact. This turns them into other important molecules. These products are determined by whether the alcohol is primary, secondary or tertiary.
The most common oxidizer is acidified potassium dichromate (VI), which is bright orange owing to the presence of chromium (Cr(VI)), which is reduced to green Cr(III) as the alcohol is oxidized on heating. The oxidizing agent is shown as “+[O]”
Primary alcohols are oxidized in two-step reactions; first forming aldehyde, then, under prolonged conditions, is oxidized further to carboxylic acid. The reaction can be stopped after aldehyde is formed and obtained using distillation. If the carboxylic acid is required, it can be obtained after continued oxidation using the apparatus for reflux.
Secondary alcohols are oxidized to the ketone by a similar process. Tertiary alcohols are not readily oxidized under comparable conditions. So there will not be a color change in potassium dichromate(VI) oxidizer when it reacts with a tertiary alcohol.
Esterification reactions
Alcohols react with carboxylic acids to form esters in a condensation reaction in which water is also a product. The reaction is an equilibrium reaction and is catalyzed by concentrated sulfuric acid. The ester has the lowest boiling point and thus can be separated using distillation. Esters have distinct smells that can be detected when they’re added to water. Esters have no free –OH bonds, so they are insoluble and form a layer on the surface of the water.
Contain an atom of fluorine, bromine, iodine, or chlorine bonded to the carbon skeleton of the molecule. Because they are saturated molecules, their reactions involve substitution (the replacement of one atom by another atom or group). However, they posses a polar bond and this makes them more reactive.
The polarity in halogenoalkanes is due to the fact that the halogen atom is more electronegative than carbon and so exerts a stronger pull on the shared electrons in the carbon-halogen bond. The halogen then gains a partial negative charge and the carbon gains a partial positive charge and is said to be electron deficient. Nucleophiles are reactants that are electron rich, as they have a lone pair and may carry a negative charge. So they are attracted to an electron deficient carbon atom in the halogenoalkane, and this leads to substitution of the halogen, known as nucleophilic substitution. –OH is a good nucleophile.
Electrophiles are reactants that are themselves electron deficient, as they have a positive charge or a partial positive charge. They are attracted to the electron-rich benzene ring, leading to electrophilic substitution reactions.
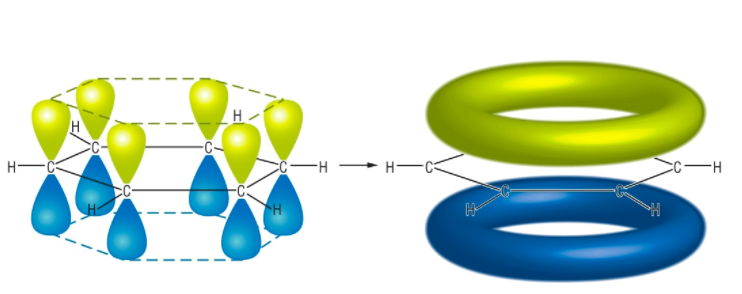
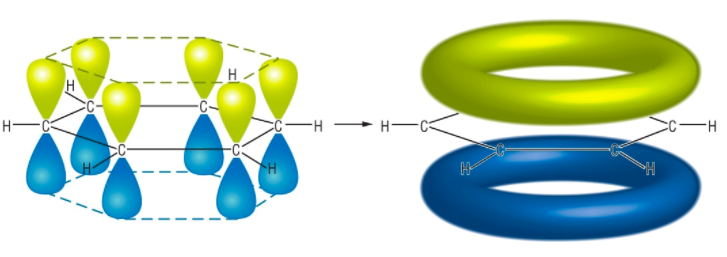
Below is the structure of a benzene ring and its delocalized electron clouds. Courtesy of www.chemhume.co.uk.



 RSS Feed
RSS Feed