Housekeeping: We will cover 9.2 and then move into Chapter 10. There will not be a quiz for Chapter 9; you missed that opportunity when you didn't come to class last year. You need to review 9.1 on your own time. We still have to get through Chapters 10, 11 and Option B.
Content Review:
Textbook: Chapter 9.2
Links: ChemGuide Redox For Dummies Half-Equations
Weebly Links: Redox Electrochemical Cells
Agenda:
Discuss 9.2.
Mission 1: Redox Review
Mission Objective. You should be able to:
1. Explain redox processes and solve problems using half-equations.
Content Review:
Textbook: Chapter 9.2
Links: ChemGuide Redox For Dummies Half-Equations
Weebly Links: Redox Electrochemical Cells
Agenda:
Discuss 9.2.
Mission 1: Redox Review
Mission Objective. You should be able to:
1. Explain redox processes and solve problems using half-equations.
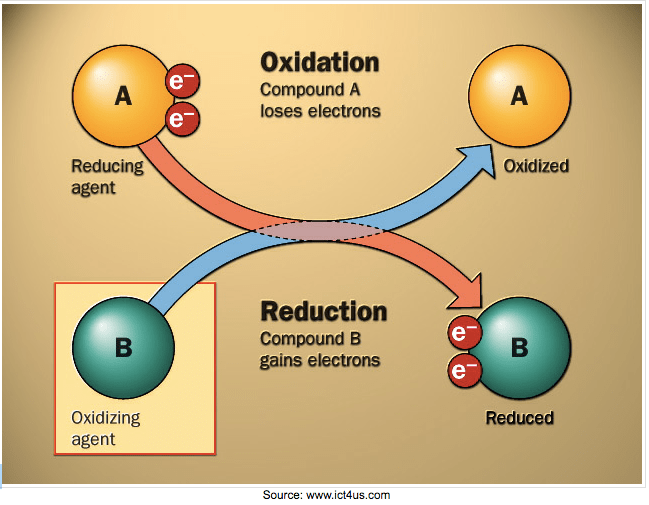
Oxidation-reduction reactions ("redox" for short) involve the transfer of electrons. There are two parts to a redox reaction. OXIDATION: when an element LOSES an electron, and REDUCTION: when an element GAINS an electron. A shortcut to remember this is: OIL RIG. (Oxidation Is Loss, Reduction Is Gain). Substances that are reduced are called oxidizing agents and substances that are oxidized are called reducing agents.
When substances are not ionic and it is harder to "see" where the electrons are going, you have to rely on electronegativity. In covalently bonded molecules, you have to look at the more electronegative of the elements (particularly groups 16 & 17). These elements have high electronegativities (ENs) because they don't want to lose the valence electrons they already have. The opposite holds true for groups 1 & 2; low ENs because they don't have a need to keep the valence electrons they already have.
Electronegativity Table (Source: www.mikeblaber.org)
The more electronegative atom is treated as if it is being reduced by gaining electrons from the other atom. The less electronegative atom is treated as if it is being oxidized by losing electrons to the other atom.
When oxidation occurs, the charge on the ion increases. The more electrons lost, the more positive the ion becomes. Manganese has two oxidation states (two types of ions). Manganese (II) has a charge of Mn+2 because two electrons were removed from the manganese atom, and manganese (IV) has a charge of Mn+4 because four electrons were removed from the atom.
When reduction occurs, the charge on the ion decreases. The more electrons gained, the smaller the charge on the ion. Using the manganese example from above, when Mn+4 is reduced by gaining two electrons, it becomes Mn+2. When Mn+2 is reduced again by gaining two more electrons, it becomes the manganese atom, Mn(s). Notice that the charge goes from +4 to +2 to 0 as the ion is reduced.
OXIDATION: Lost electrons are written on the PRODUCT side (to the right of the arrow).
REDUCTION: Gained electrons are written on the REACTANT side (to the left of the arrow).
Oxidation and reduction are PROCESSES, and substances (elements or compounds) are either REDUCING AGENTS or OXIDIZING AGENTS. Oxidizing agents oxidize other substances by accepting its electrons. Strong oxidizing agents: groups 16 & 17 (&15). Reducing agents reduce other substances by supplying electrons to the substance being reduced. Strong reducing agents: groups 1 & 2 (and 3-13).
Here is an EduCreate video giving you some examples to work with.
This is a link to some videos that walk you through oxidation numbers, redox reactions, and half-reactions.
Mission 2: Electrochemistry
Mission Objectives. You should be able to...
1. Construct a voltaic cell and an eletrolytic cell and determine electron flow.
2. Define "cathode" and "anode" in terms of electron flow and redox process.
3. Describe the difference between a voltaic cell and an electrochemical cell.
4. Determine how to annotate a diagram of both types of cells.
When substances are not ionic and it is harder to "see" where the electrons are going, you have to rely on electronegativity. In covalently bonded molecules, you have to look at the more electronegative of the elements (particularly groups 16 & 17). These elements have high electronegativities (ENs) because they don't want to lose the valence electrons they already have. The opposite holds true for groups 1 & 2; low ENs because they don't have a need to keep the valence electrons they already have.
Electronegativity Table (Source: www.mikeblaber.org)
The more electronegative atom is treated as if it is being reduced by gaining electrons from the other atom. The less electronegative atom is treated as if it is being oxidized by losing electrons to the other atom.
When oxidation occurs, the charge on the ion increases. The more electrons lost, the more positive the ion becomes. Manganese has two oxidation states (two types of ions). Manganese (II) has a charge of Mn+2 because two electrons were removed from the manganese atom, and manganese (IV) has a charge of Mn+4 because four electrons were removed from the atom.
When reduction occurs, the charge on the ion decreases. The more electrons gained, the smaller the charge on the ion. Using the manganese example from above, when Mn+4 is reduced by gaining two electrons, it becomes Mn+2. When Mn+2 is reduced again by gaining two more electrons, it becomes the manganese atom, Mn(s). Notice that the charge goes from +4 to +2 to 0 as the ion is reduced.
OXIDATION: Lost electrons are written on the PRODUCT side (to the right of the arrow).
REDUCTION: Gained electrons are written on the REACTANT side (to the left of the arrow).
Oxidation and reduction are PROCESSES, and substances (elements or compounds) are either REDUCING AGENTS or OXIDIZING AGENTS. Oxidizing agents oxidize other substances by accepting its electrons. Strong oxidizing agents: groups 16 & 17 (&15). Reducing agents reduce other substances by supplying electrons to the substance being reduced. Strong reducing agents: groups 1 & 2 (and 3-13).
Here is an EduCreate video giving you some examples to work with.
This is a link to some videos that walk you through oxidation numbers, redox reactions, and half-reactions.
Mission 2: Electrochemistry
Mission Objectives. You should be able to...
1. Construct a voltaic cell and an eletrolytic cell and determine electron flow.
2. Define "cathode" and "anode" in terms of electron flow and redox process.
3. Describe the difference between a voltaic cell and an electrochemical cell.
4. Determine how to annotate a diagram of both types of cells.
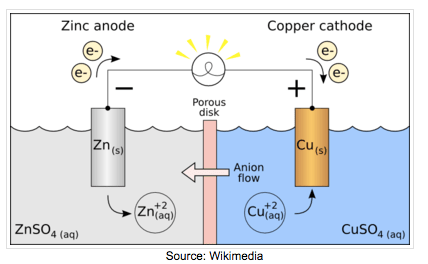
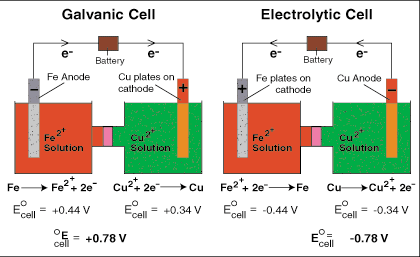
Electrochemistry transfers the chemical energy of a redox reaction into electrical energy. Recall what happens in redox reactions. When the substances involved in oxidation and reduction half–reactions are physically separated, it is called an electrochemical cell. Each half reaction occurs on the surface of an electrically conductive solid called an electrode, and each electrode is immersed in a solution containing the ions needed for the half–reaction.
The electrodes are connected by a wire so that electrons can move from the oxidation half–reaction to the reduction half–reaction. The solutions are connected by a salt bridge so that ions can move between solutions. In an electrochemical cell, the chemical potential energy can be harnessed as the substances undergoing oxidation push electrons through the wire to the substances undergoing reduction.
The electrodes are connected by a wire so that electrons can move from the oxidation half–reaction to the reduction half–reaction. The solutions are connected by a salt bridge so that ions can move between solutions. In an electrochemical cell, the chemical potential energy can be harnessed as the substances undergoing oxidation push electrons through the wire to the substances undergoing reduction.
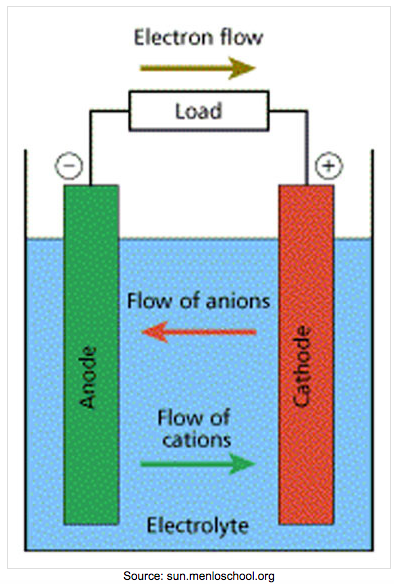
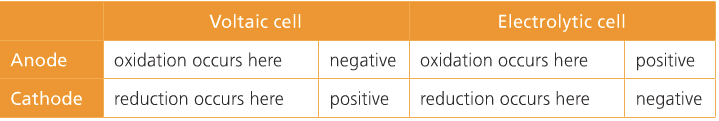
| Voltaic Cell (also called galvanic cell) In a voltaic cell, electrons are spontaneously emerging at the cathode so that reduction can occur. Electrons are the source of potential in a voltaic cell. The electrons are pushed from the anode towards the cathode, supplying the resistance. Since the electrons leave the anode, it is designated with a negative sign. The electrons build up at the cathode, therefore, the cathode is designated with a positive sign. Anode: The anode is the electrode in the electrochemical cell where oxidation occurs. Anions are attracted to the anode because it is positively charged. This is because it is losing electrons in the half-reaction. |
Cathode:
The cathode is the electrode in the electrochemical cell where reduction occurs. Cations are attracted to the cathode because it is negatively charged. This is because it is gaining electrons in the half reaction.
Salt Bridge:
The salt bridge completes the circuit by moving ions into the solutions to maintain electric neutrality without mixing them.
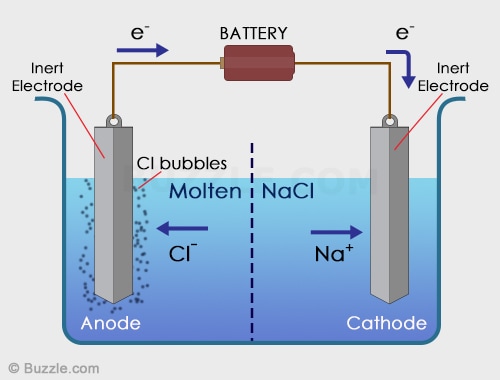
Electrolytic Cell
In an electrolytic cell, non-spontaneous reactions are forced to occur using electricity. Non-spontaneous reactions do not follow the path of least resistance. The electrons have to be driven using a current. Electroplating is a process that coats a material with a layer of metal based on metallic activity. Below is an image of an electrolytic cell. These take place in one beaker.
Image from buzzle.com
The cathode is the electrode in the electrochemical cell where reduction occurs. Cations are attracted to the cathode because it is negatively charged. This is because it is gaining electrons in the half reaction.
Salt Bridge:
The salt bridge completes the circuit by moving ions into the solutions to maintain electric neutrality without mixing them.
Electrolytic Cell
In an electrolytic cell, non-spontaneous reactions are forced to occur using electricity. Non-spontaneous reactions do not follow the path of least resistance. The electrons have to be driven using a current. Electroplating is a process that coats a material with a layer of metal based on metallic activity. Below is an image of an electrolytic cell. These take place in one beaker.
Image from buzzle.com
Voltaic cells are typically used to produce electrical energy. Batteries are a series of voltaic cells. Electrolytic cells use electricity to bring about a redox reaction that would normally be non-spontaneous. In other words, low-energy reactants become high-energy products.
Charges on the electrodes are inverted in electrolytic cells compared to voltaic cells. This is because the nature of a redox reaction defines the electrode: oxidation always occurs at the anode and reduction always occurs at the cathode. So electrons flow from anode to cathode.
Charges on the electrodes are inverted in electrolytic cells compared to voltaic cells. This is because the nature of a redox reaction defines the electrode: oxidation always occurs at the anode and reduction always occurs at the cathode. So electrons flow from anode to cathode.
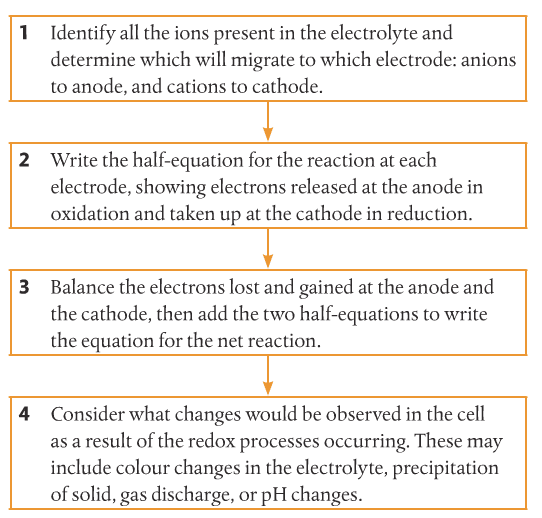
Mission 3: Hot Salt.
Mission Objective. You should be able to...
1. Use a series of steps to predict products in the electrolysis of molten salts.
Mission Objective. You should be able to...
1. Use a series of steps to predict products in the electrolysis of molten salts.
Let's Practice!!!
 RSS Feed
RSS Feed