Content Review:
Textbook: Chapter 9
Links: ChemGuide Redox For Dummies Half-Equations
Agenda:
1. Electrochemical Cells
Mission 1: Battery Powered!
Mission Objectives. You should be able to:
1. Draw, construct and annotate a voltaic cell.
2. Explain how a redox reaction is used to produce electricity in a voltaic cell.
3. Describe the relationship between solution concentration and voltage.
Electrochemistry transfers the chemical energy of a redox reaction into electrical energy. Recall what happens in redox reactions.
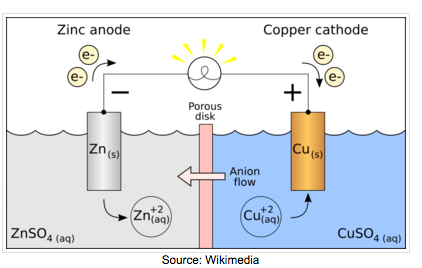
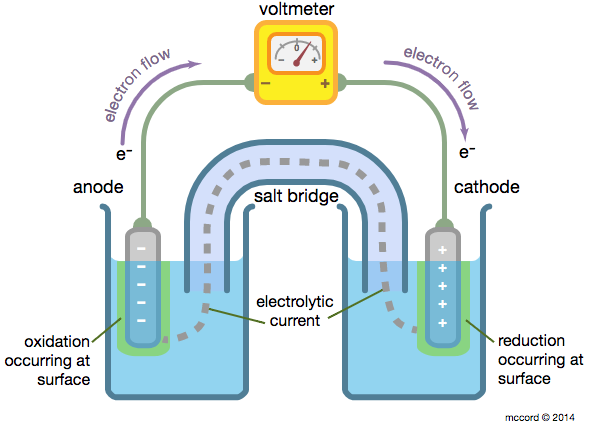
When the substances involved in oxidation and reduction half–reactions are physically separated, it is called an electrochemical cell. Each half reaction occurs on the surface of an electrically conductive solid called an electrode, and each electrode is immersed in a solution containing the ions needed for the half–reaction.
The electrodes are connected by a wire so that electrons can move from the oxidation half–reaction to the reduction half–reaction. The solutions are connected by a salt bridge so that ions can move between solutions. In an electrochemical cell, the chemical potential energy can be harnessed as the substances undergoing oxidation push electrons through the wire to the substances undergoing reduction.
Voltaic cells are typically used to produce electrical energy. Batteries are voltaic cells. Electrolytic cells use electricity to bring about a redox reaction that would normally be non-spontaneous. In other words, low-energy reactants become high-energy products.
Rechargeable batteries: Spontaneous redox reactions eventually deplete the electrons available at the anode (e.g. causing a battery to become “dead”). The redox reactions can be reversed by a non-spontaneous reaction. More on batteries.
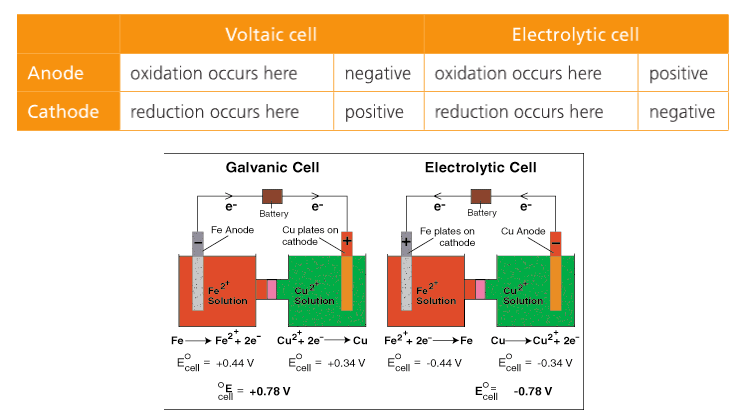
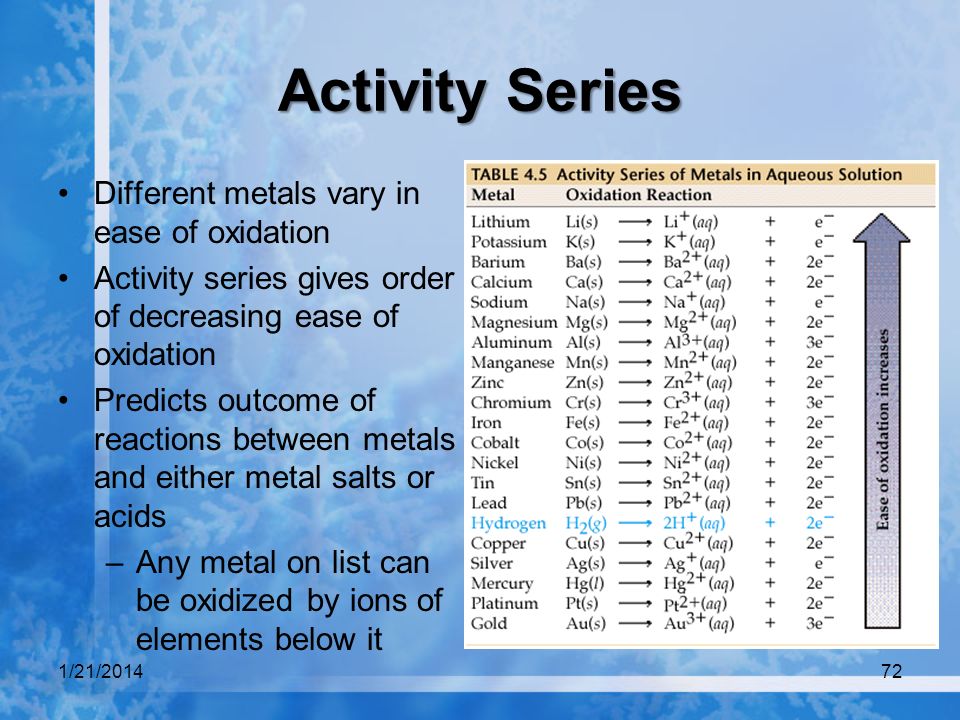
In a voltaic cell, the cathode is the positive electrode and the anode is the negative electrode. Oxidation takes place at the anode and reduction takes place at the cathode. Electrons are driven spontaneously from the anode to the cathode. Metal/metal ion electrodes consist of a bar of metal dipped into a solution containing cations of the same metal. Typical examples include: Fe(s)|Fe2+(aq), Zn(s)|Zn2+(aq), and Cu(s)|Cu2+(aq). The vertical line represents the phase boundary.
Salt bridges are used to connect the half cells. They serve three functions: (1) prevents mixing of the solutions but allows oxidation and reduction to occur, (2) provides a path for the migration of the cations and anions in the cell, and (3) reduces the liquid-junction potential.
Read on pages 228-229 about the construction of a Daniell Cell. This is what you'll be constructing on Friday.
Mission 2: Electrolytic Cells
Mission Objectives. You should be able to...
1. Draw and annotate an electrolytic cell.
2. Describe the flow of electrons in an electrolytic cell.
3. Deduce the electrolysis of a molten salt.
4. Compare a voltaic cell with an electrolytic cell.
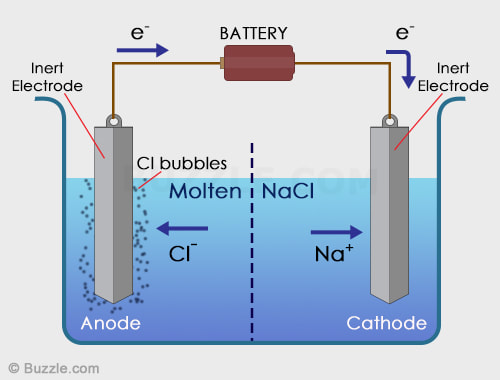
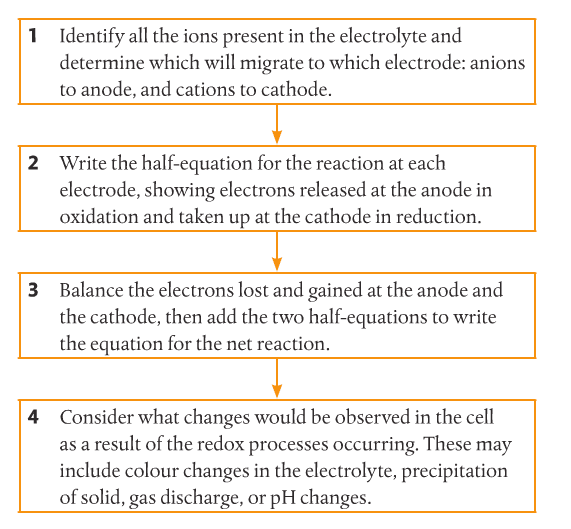
Electrolytic Cells. Electrolysis is the process by which electrical energy is used to drive a non-spontaneous chemical reaction. An electrolytic cell consists of a single container, two electrodes, a solution (electrolyte), and a battery to drive the electrons in reverse. In an electrolytic cell, the cathode is the negative electrode and the anode is the positive electrode. Electroplating is a process that coats a material with a layer of metal based on metallic activity. Below is an image of an electrolytic cell. These take place in one beaker.
Courtesy of buzzle.com
Below is a summary from the Pearson textbook.
Mission Objective. You should be able to...
1. Use a series of steps to predict products in the electrolysis of molten salts.



 RSS Feed
RSS Feed