I am going to combine the Chapter 5 exam with Chapter 6, so you will take just one test. It is easier that way and since your exam is scheduled for May 10, you have plenty of time to master the content in Chapter 6.
Content Review
Textbook: Chapter 6
Mad!Science! Links: Kinetics
Agenda:
Complete the tasks found here and in hard copy.
Lesson Objectives: You should be able to:
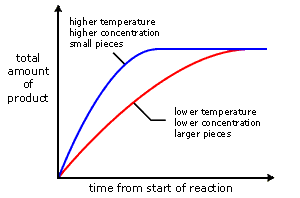
1. Explain the factors that affect rates of reaction.
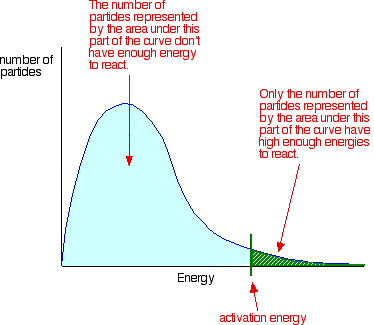
2. Construct a Maxwell-Boltzmann energy distribution curve to account for the probability of successful collisions and factors that affect them, including the effect of a catalyst.
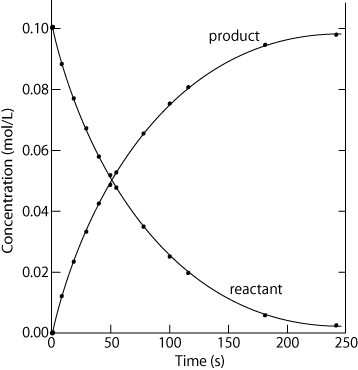
3. Investigate rates of reaction experimentally and evaluate the results.
4. Sketch and explain energy profiles with and without catalysts.
Mission 1: Reaction Mechanisms. The mechanism of a chemical reaction is the series of events that takes place as reactants are converted into products. You can read more about it on ChemWiki. The first video, from Brightstorm, introduces the concept of reaction mechanisms. The second video, which is IB, goes into detail about determining rate laws, which we will deal with soon enough.
 RSS Feed
RSS Feed