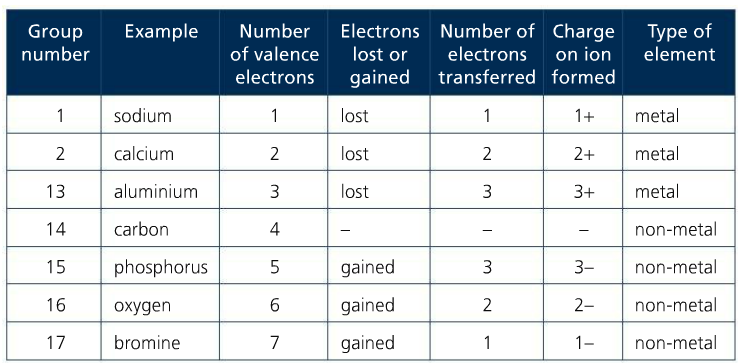
p. 132 #14-17, p. 136 #18-20, p. 138 #21, p. 143 #23
These problems are going into the gradebook as an assignment and I'll be looking for completion on Wednesday & Thursday.
Agenda:
1. VSEPR Theory & Molecular Geometry.
Lesson Objectives. By the end of this lesson, you should be able to:
1. Explain VSEPR Theory.
2. Draw Lewis structures of covalent compounds using VSEPR Theory.
3. Use VSEPR Theory to predict molecular geometry of different compounds
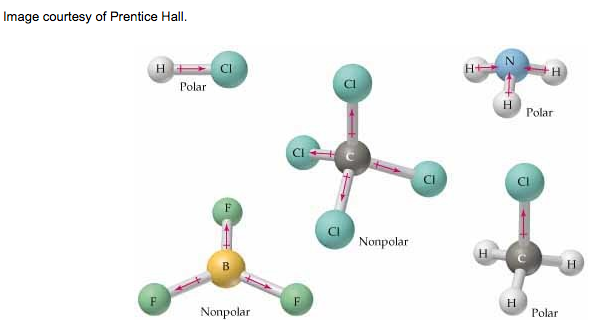
4. Use VSEPR Theory to predict molecular polarity.
5. Draw and describe resonance structures of different compounds, such as benzene, carbonate, and ozone.
6. Explain the properties of giant covalent compounds in terms of their structures,
Content Review:
Links: VSEPR Theory Molecular Geometry
Textbook Readings: Chapter 4.
Student Missions:
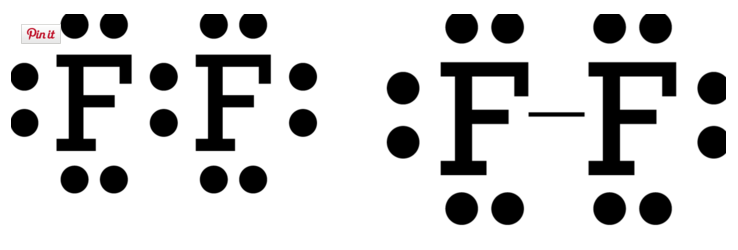
Mission 1: What Molecules Look Like. VSEPR stands for Valence Shell Electron Pair Repulsion. This theory helps us to predict the shape of molecules using their valence electrons. There are six steps to drawing Lewis structures using the VSEPR theory. Page 130 lists the six rules. Mr. Anderson walks you through the process. We will work some practice problems in class.
Mission 2: Keeping Things Straight. Remember that the book (and therefore the exams) will refer to shared pairs of electrons as electron domains. Here is what we know so far:
Molecules (or species) with two electron domains tend to be linear with bond angles of 180 degrees.
Species with three electron domains tend to be trigonal planar with bond angles of 120 degrees. But if one of the electron domains is a lone pair (or unshared pair [two dots]), it doesn't show in the overall shaped. This results in species that are bent and have bond angles of 117 degrees.
Species with four electron domains tend to be tetrahedral and have bond angles of 109.5 degrees.
Pages 136-7 provide excellent summary tables of these shapes.
Mission 3: Resonance. Delocalization is when bonding electrons are not restricted to specific positions in molecules. They can spread out and give greater stability to thee molecule or ion. This happens frequently in molecules when there is more than one possible position for a double bond within a molecule. Example: Ozone
As a result, molecules like these demonstrate resonance. The Lewis structures are called resonance structures, as there are multiple versions of where the delocalized electrons can be. See the carbonate example on page 140. Also look at the benzene example on page 141. This is a specific example you are expected to know.
Resonance gives special properties to the structures where it occurs. It affects bond lengths and strengths, which influence reactivity. Acid and base strengths can be explained by resonance.
Play with this simulation.
Mission 4:

 RSS Feed
RSS Feed