Housekeeping: We should finish Section 9.1 early next week. So you guys need to pick a quiz date. Then we will move on to electrochemical cells. This section will require some lab time.
Reem, I have yet to receive your IA draft. The final due date for all science IAs is December 15th.
Content Review:
Textbook: Chapter 9
Links: ChemGuide Redox For Dummies Half-Equations
Agenda:
1. Continue working with half-reactions
2. Discussion of oxidants and reductants
Mission 1: Which Way You Swing?
Mission Objective. You should be able to:
1. Contrast oxidizing agent and reducing agent with oxidation and reduction.
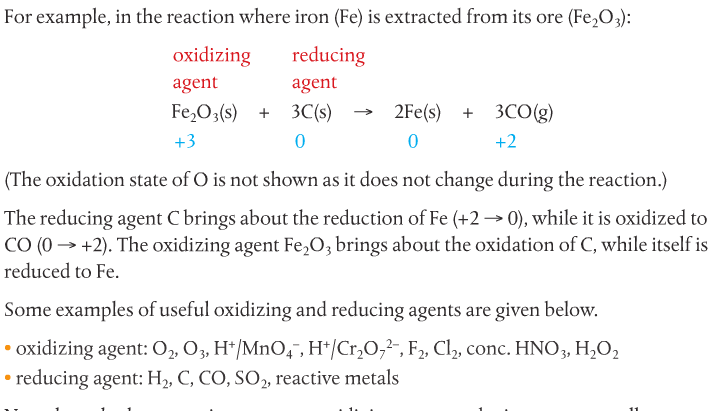
The reactant that accepts electrons is called the oxidizing agent (or oxidant). It brings about the oxidation of the other reactant. The reactant that supplies the electrons is called the reducing agent (or reductant). See the example below, courtesy of your textbook.
Reem, I have yet to receive your IA draft. The final due date for all science IAs is December 15th.
Content Review:
Textbook: Chapter 9
Links: ChemGuide Redox For Dummies Half-Equations
Agenda:
1. Continue working with half-reactions
2. Discussion of oxidants and reductants
Mission 1: Which Way You Swing?
Mission Objective. You should be able to:
1. Contrast oxidizing agent and reducing agent with oxidation and reduction.
The reactant that accepts electrons is called the oxidizing agent (or oxidant). It brings about the oxidation of the other reactant. The reactant that supplies the electrons is called the reducing agent (or reductant). See the example below, courtesy of your textbook.
More reactive metals are strong reducing agents. Metals have a tendency to lose electrons and form cations, so they become reducing agents. The more reactive the metal is, the stronger its reducing ability. If you reference the activity series of metals in your Data Booklet, you can identify which metals are likely to be strong reductants and which ones are weak.
More reactive nonmetals are strong oxidizing agents. Nonmetals have a tendency to gain electrons and form anions, so they become oxidizing agents. The more reactive the nonmetal is, the stronger its oxidizing ability. Examine the activity series of nonmetals to identify strong oxidants.
Mission 2: *sigh* More With Equations.
Mission Objective. You should be able to:
1. Write and balance redox equations using half-equations.
More reactive nonmetals are strong oxidizing agents. Nonmetals have a tendency to gain electrons and form anions, so they become oxidizing agents. The more reactive the nonmetal is, the stronger its oxidizing ability. Examine the activity series of nonmetals to identify strong oxidants.
Mission 2: *sigh* More With Equations.
Mission Objective. You should be able to:
1. Write and balance redox equations using half-equations.
The rules for writing and balancing redox half-reactions are on pages 274-5. I've included the summary here. We will practice writing and balancing redox equations in the blue boxes on pages 275-6. We will take our time with this, depending on your needs.
Homework: Complete the blue box problems.
Mission 3: Redox Titrations.
Mission Objectives. You should be able to:
1. Solve redox titration problems.
2. Explain the Winkler Method of measuring biochemical oxygen demand.
Redox titrations are carried out just like acid-base titrations. They are commonly used in the food and beverage industry, in pharmaceuticals and in water and environmental analysis. Khan Academy describes this in detail in the below video.
Mission 3: Redox Titrations.
Mission Objectives. You should be able to:
1. Solve redox titration problems.
2. Explain the Winkler Method of measuring biochemical oxygen demand.
Redox titrations are carried out just like acid-base titrations. They are commonly used in the food and beverage industry, in pharmaceuticals and in water and environmental analysis. Khan Academy describes this in detail in the below video.
In your book, two equations are provided. The first one is the analysis of iron with manganate (VII). Potassium permanganate acts as an oxidizing agent that oxidizes iron (II) ions into iron (III) ions. The manganate ion is reduced to manganese (II). The color changes from deep purple to colorless, which signals the equivalence point.
The second equation is the iodine-thiosulfate reaction. Iodine is reduced from an initial reaction, and then is titrated with sodium thiosulfate and starch is used as an indicator. The solution is blue, but as iodine is reduced to iodide (I-), the blue color disappears.
Below is an explanation of the Winkler Method. In brief, the Winkler method calculates biological oxygen demand (BOD). It is a series of redox reactions; the steps of which are on page 285.
The second equation is the iodine-thiosulfate reaction. Iodine is reduced from an initial reaction, and then is titrated with sodium thiosulfate and starch is used as an indicator. The solution is blue, but as iodine is reduced to iodide (I-), the blue color disappears.
Below is an explanation of the Winkler Method. In brief, the Winkler method calculates biological oxygen demand (BOD). It is a series of redox reactions; the steps of which are on page 285.
Homework: Practice problems handout.

 RSS Feed
RSS Feed