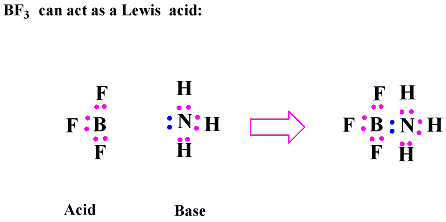
In the example below, BF3 is behaving as a Lewis acid. Ammonia, NH3, is behaving as a Lewis base. The lone pair of electrons on nitrogen moves to boron and the result is a coordinate covalent bond.
|
The Lewis acid-base theory extends the definition of an acid and base from [H+] and [OH-]. Lewis' theory used electrons instead of protons. His theory says that bases are electron donors and acids are electron acceptors. When a base donates a pair of electrons to an acid, a coordinate covalent bond is formed. In the above video, ChemisNATE illustrates via Lewis structures how electrons are transferred from bases to acids. You have to remember how to draw Lewis Structures for simple compounds.
In the example below, BF3 is behaving as a Lewis acid. Ammonia, NH3, is behaving as a Lewis base. The lone pair of electrons on nitrogen moves to boron and the result is a coordinate covalent bond.
1 Comment
6/24/2018 01:34:35 am
Thank you for sharing this information regarding the acid and the base. The first time I saw this blog, I have come to think of a way to teach this to my class while having fun and at the same time, to encourage them to really study this matter. Teenagers were so hard to be encourage regarding studying but once you got their secrets on how to be conscious or alert to the matter, they will definitely remember the lesson.
Reply
Leave a Reply. |
Archives
February 2020
|
 RSS Feed
RSS Feed