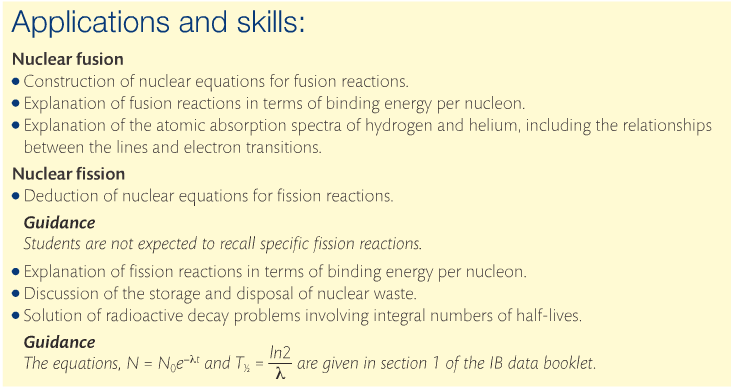
C3: Nuclear Fusion & Fission
There is an abundance of fuel for nuclear fusion and the lack of waste makes it an attractive prospect for energy generation. However, there are technological issues involved. Fusion takes place at high temperatures and there is no material that can contain it. In the sun, hydrogen converts to helium, but there is a difference in the mass of a helium nucleus and the sum of the masses of 2 protons and 2 neutrons. This is known as mass defect. The missing mass is converted directly into energy, which can be predicted using E = mc2. This is energy calculated per atom, and the unit is the electron volt (eV).
Fusion of lighter elements into heavier ones increases the binding energy and the mass defect is converted into energy. The heavier transuranium elements (Z=92+) can undergo fission to form lighter nuclei. The binding energy of the two lighter elements is greater than the binding energy of a uranium isotope and therefore, the mass defect is converted into energy. Controlled nuclear fission is the process that powers nuclear plants nowadays.
Read about critical mass on page 669.
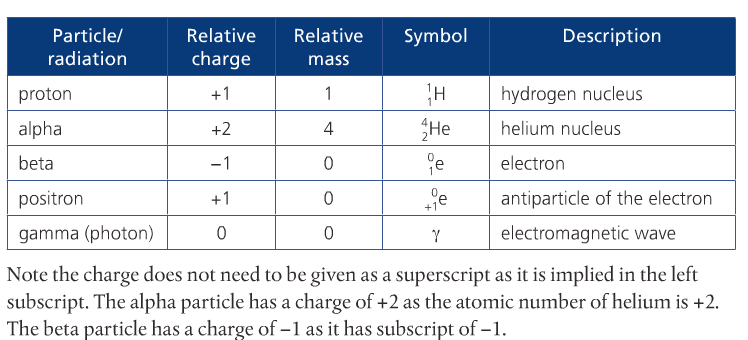
Some heavier atoms are radioactive, which means they undergo spontaneous decay to produce daughter products, releasing alpha, beta, and/or gamma radiation in the process. Radioactive decay is a first-order reaction, which means that they have a constant half-life. The half-life refers to the time it takes for one half of the number of atoms in a sample to decay. There is an equation for half-life in your text on page 671.
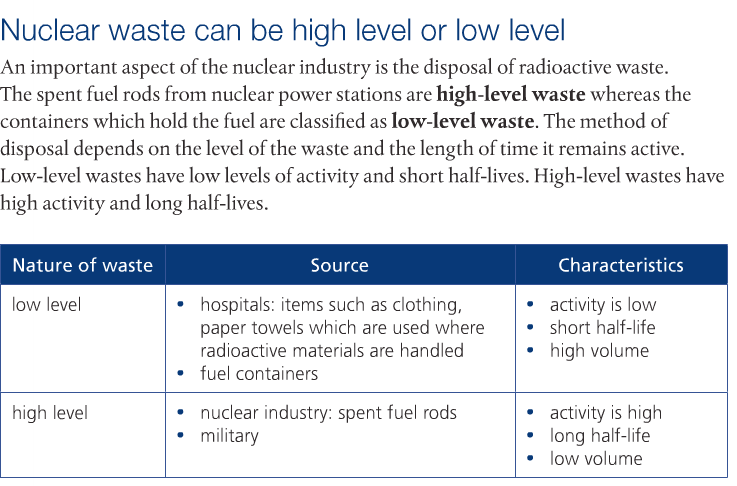
Uranium-235 is the only natural occurring uranium that is fissionable. However, there is only 0.72% of naturally occurring U-235. In order to obtain fissile material, U-235 must be enriched so that the percentage of U-235 is large enough. To do this, the U-235 isotope must be separated from the U-238 isotope. Pages 704-705 goes into detail how this is done.
Graham's Law of Effusion allows you to calculate the relative rates of diffusion of the uranium hexafluoride.


 RSS Feed
RSS Feed