Housekeeping: Good morning. Welcome to The!Mad!Scientist!
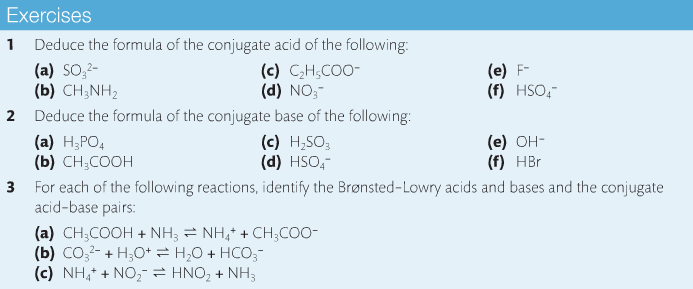
Content Review:
Textbook:
Links:
Agenda:
1. Welcome & introduction
2. Navigating the website
3. Catching up
4. Acid/base theory
5. Practice with conjugate acids & bases identification.
6. Discover properties of acids and bases.
7. The pH scale
Mission 1: Acid-Base Theories.
Mission Objectives. You should be able to:
1. Define, compare and contrast acid/base theories.
2. Explain why Arrhenius' model had to be replaced.
3. Identify conjugate acid/base pairs.
We will begin with the homie Richard Thornley.
Content Review:
Textbook:
Links:
Agenda:
1. Welcome & introduction
2. Navigating the website
3. Catching up
4. Acid/base theory
5. Practice with conjugate acids & bases identification.
6. Discover properties of acids and bases.
7. The pH scale
Mission 1: Acid-Base Theories.
Mission Objectives. You should be able to:
1. Define, compare and contrast acid/base theories.
2. Explain why Arrhenius' model had to be replaced.
3. Identify conjugate acid/base pairs.
We will begin with the homie Richard Thornley.
There are three known acid-base theories: Arrhenius, Bronsted-Lowry, and Lewis. Arrhenius's theory is very simple: Acids disassociate in water to form H+ ions and bases disassociate to form OH- ions. Bronsted-Lowry (BL)'s definition is more inclusive. Acids are proton donors and bases are proton acceptors. Lewis' definition focuses on the behavior of electrons. Acids accept electron pairs and bases donate electron pairs.
We will focus mainly on Bronsted-Lowry's theory. This leads us to the formation of conjugate acids and bases.
We will focus mainly on Bronsted-Lowry's theory. This leads us to the formation of conjugate acids and bases.
Acids react to form bases and bases react to form acids. The acid-base pairs related to each other like this are called conjugate acid-base pairs and differ by a proton. Substances that can act as an acid and a base are called amphoteric. Water is a good example, as is the HCO3- ion.
A good example is when water becomes the hydronium ion by accepting a proton.
H2O(l) + H+(aq) --> H3O+(aq)
This makes H2O/H3O+ a conjugate acid-base pair.
A good example is when water becomes the hydronium ion by accepting a proton.
H2O(l) + H+(aq) --> H3O+(aq)
This makes H2O/H3O+ a conjugate acid-base pair.
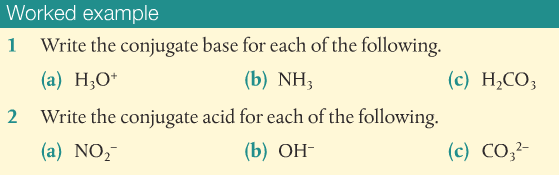
Let's practice!!!
Homework: See below.
Mission 2: Equal yet Opposite.
Mission Objectives. You should be able to:
1. List and describe the properties of acids and bases.
2. Identify the acids and bases needed to make different salts.
3. Explain the necessity of indicators.
Acids and bases have different characteristics. Acids taste sour and are sometimes sticky. They are corrosive. Acids turn blue litmus red, yellow methyl orange into red, and are colorless in the presence of phenolphthalein. Bases taste bitter and are usually slippery. They are caustic. Bases turn red litmus blue and are pink in the presence of phenolphthalein.
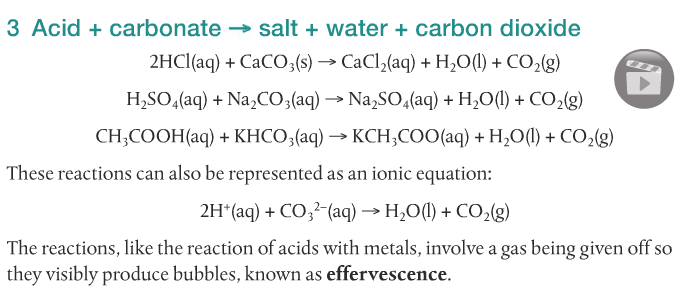
Acids react with bases, metals and carbonates to form salts. The following video shows five different reactions of acids and their products.
Mission Objectives. You should be able to:
1. List and describe the properties of acids and bases.
2. Identify the acids and bases needed to make different salts.
3. Explain the necessity of indicators.
Acids and bases have different characteristics. Acids taste sour and are sometimes sticky. They are corrosive. Acids turn blue litmus red, yellow methyl orange into red, and are colorless in the presence of phenolphthalein. Bases taste bitter and are usually slippery. They are caustic. Bases turn red litmus blue and are pink in the presence of phenolphthalein.
Acids react with bases, metals and carbonates to form salts. The following video shows five different reactions of acids and their products.
We will focus on the reactions of acids with metals, acids with bases, and acids with carbonates.
It is my plan to have you guys doing your own variations of these demonstrations later in the week. Whenever possible, it's always better to do it yourself. You may get different results and will have to explain why. Don't be afraid. We will be titrating next week, hopefully.
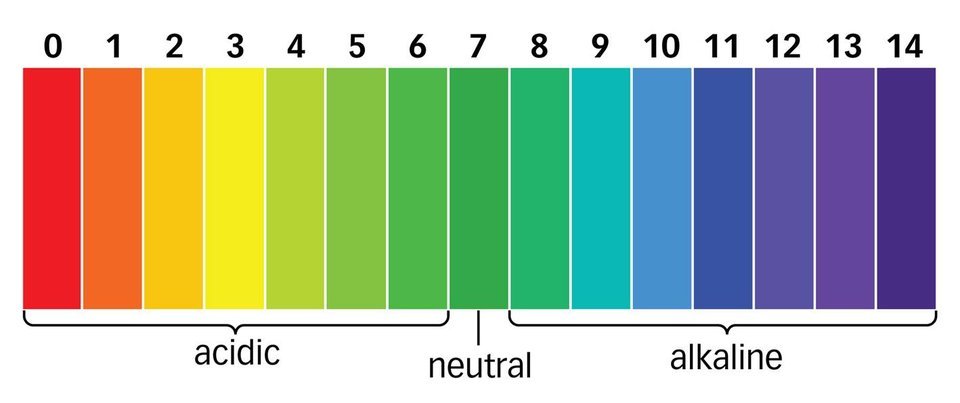
Indicators are chemical detectors. Acids and bases change color depending on which indicator is used. The most common indicator is litmus (turns red in the presence of acid and blue in the presence of base). Phenolphthalein turns pink in the presence of a base, but is colorless when combined with an acid. Universal indicator is a combination of many other indicators that turns a variety of colors depending on the concentration of H+ in solution.
Image courtesy of https://tackk.com/qbel4z
Indicators are chemical detectors. Acids and bases change color depending on which indicator is used. The most common indicator is litmus (turns red in the presence of acid and blue in the presence of base). Phenolphthalein turns pink in the presence of a base, but is colorless when combined with an acid. Universal indicator is a combination of many other indicators that turns a variety of colors depending on the concentration of H+ in solution.
Image courtesy of https://tackk.com/qbel4z
Homework: See below
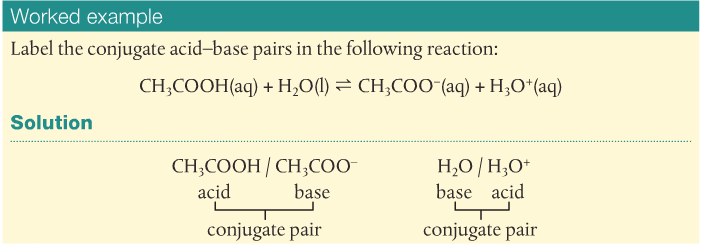
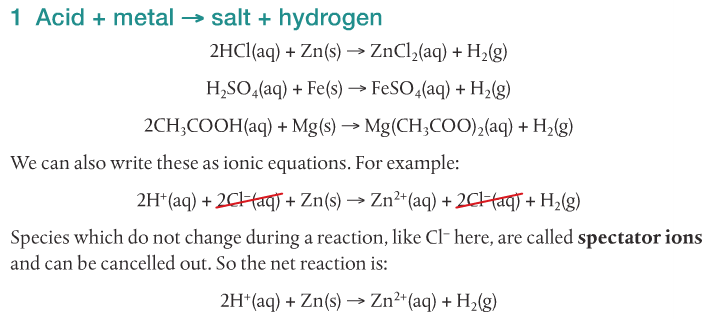
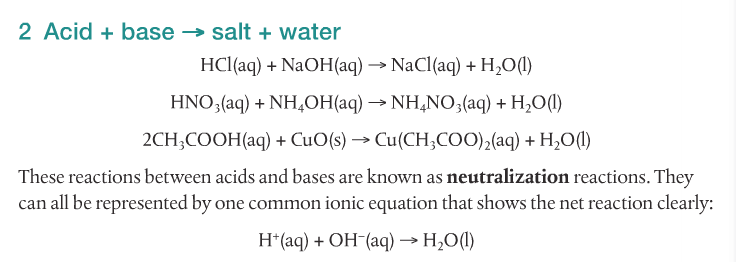

 RSS Feed
RSS Feed