Housekeeping: Need to see progress on IA topics. If you need to speak to me outside of class, I'm available M-W between 10:35 - 1:00.
We have completed Chapter 4. Your exam is scheduled for October 12. We are now going to go back to Chapter 1, which is quantitative chemistry. There is a lot in Chapter one, so I anticipate us finishing it sometime in November.
Today you guys will learn about the five kinds of chemical reactions and you will play around with elements and compounds.
Agenda:
1. Introduction to chemical equations
2. The mole concept
Content Review:
Links: Particulate Nature of Matter Chemical Reactions
Mission 1: Breaking it Down, Atomic Style.
Mission Objectives. You should be able to...
1. Deconstruct and explain the components of a chemical equation.
2. Understand the difference between reactants and products.
3. Explain what state symbols are and how they are used in a chemical equation.
4. Determine how to balance a chemical equation.
5. Solve conservation of mass in chemical equations.
6. List the five kinds of chemical reactions.
I will show you some chemical equations and we will deconstruct them. Here are some notes to get you started. Don't worry; we're just tackling the first page.
State symbols are used in chemical equations to denote the physical state of the substances before and after reacting. The BBC has a nice little Bitesize denoting all four state symbols. Be sure you know how to write them and what they mean.
BALANCE, BABY, BALANCE!!! The Law of Conservation of Mass says that mass is always conserved. So what does this mean for chemical reactions? It means adding atoms and ions, of course. We need a worksheet to practice with, so I have provided one.
We have completed Chapter 4. Your exam is scheduled for October 12. We are now going to go back to Chapter 1, which is quantitative chemistry. There is a lot in Chapter one, so I anticipate us finishing it sometime in November.
Today you guys will learn about the five kinds of chemical reactions and you will play around with elements and compounds.
Agenda:
1. Introduction to chemical equations
2. The mole concept
Content Review:
Links: Particulate Nature of Matter Chemical Reactions
Mission 1: Breaking it Down, Atomic Style.
Mission Objectives. You should be able to...
1. Deconstruct and explain the components of a chemical equation.
2. Understand the difference between reactants and products.
3. Explain what state symbols are and how they are used in a chemical equation.
4. Determine how to balance a chemical equation.
5. Solve conservation of mass in chemical equations.
6. List the five kinds of chemical reactions.
I will show you some chemical equations and we will deconstruct them. Here are some notes to get you started. Don't worry; we're just tackling the first page.
State symbols are used in chemical equations to denote the physical state of the substances before and after reacting. The BBC has a nice little Bitesize denoting all four state symbols. Be sure you know how to write them and what they mean.
BALANCE, BABY, BALANCE!!! The Law of Conservation of Mass says that mass is always conserved. So what does this mean for chemical reactions? It means adding atoms and ions, of course. We need a worksheet to practice with, so I have provided one.
Now let's go backwards a bit. What is the difference between substances, mixtures, elements and compounds? Paul Anderson of Bozeman Science gives you an overview of matter. What is it, what does it do, where you can find it, all that information.
So what's the deal with all this stuff? It's so much, it's too big...why is distinguishing it so important? Why does it matter (get it?)? Well, take a look here and then we'll go here to find out why. And while we're at it, we shall bask in the glory that is the Periodic Table of Elements.
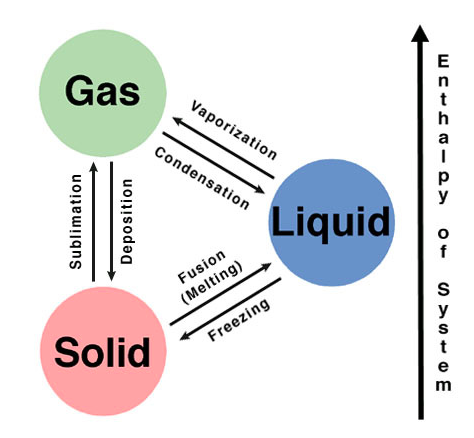
Now you know the basic three states of matter. Did you know they could change into one another? Take a look at this graphic and try to explain what you're seeing using water as your example. Which set of triangles represent endothermic changes (energy absorbed)? Which set of triangles represent exothermic changes (energy released)?
So what's the deal with all this stuff? It's so much, it's too big...why is distinguishing it so important? Why does it matter (get it?)? Well, take a look here and then we'll go here to find out why. And while we're at it, we shall bask in the glory that is the Periodic Table of Elements.
Now you know the basic three states of matter. Did you know they could change into one another? Take a look at this graphic and try to explain what you're seeing using water as your example. Which set of triangles represent endothermic changes (energy absorbed)? Which set of triangles represent exothermic changes (energy released)?
Mission 2: The Notorious B.I.G. in chemistry
Mission Objectives. You should be able to...
1. Understand the nature of the mole aka Avogadro's Number
2. Calculate mole conversions
Mission Objectives. You should be able to...
1. Understand the nature of the mole aka Avogadro's Number
2. Calculate mole conversions
Okay, so now that we have some understanding of how massive a mole is, let's get into what it's used for. Paul Anderson of Bozeman Science brings it in only the way he can. Then we will practice solving mole conversion problems.
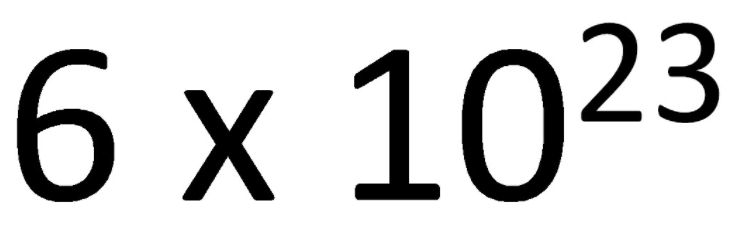
 RSS Feed
RSS Feed