Housekeeping: We are now covering Chapter 7, Equilibrium. The exam for chapters 6 & 7 are on April 6 for SL and April 16 for HL
Content Review:
Textbook: Chapter 7
Links: IB Chemistry Home Notes
Mission 1: Balance, Baby...Balance!
Mission Objectives: You should be able to:
1. Describe characteristics of chemical and physical systems at equilibrium.
2. Deduce the equilibrium constant (Kc) from an equation.
3. Explain Le Chatelier's Principle in your own words.
4. Define and explain reaction quotient.
5. Explain what it means when reactions must shift either to the left or the right.
6. Describe the effects of changing concentration, temperature and pressure on reactions at equilibrium.
7. Describe what happens when a catalyst is added to a reaction at equilibrium.
8. Explain what the Haber Process and the Contact Process are, and their significance.
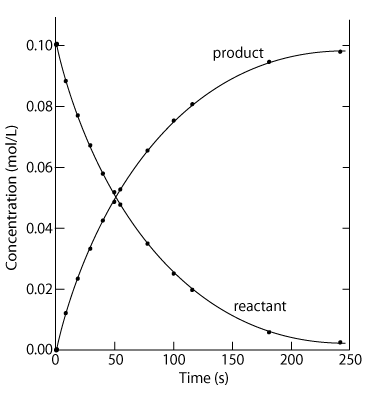
Many chemical reactions are reversible and exist in a state of equilibrium. Equilibrium occurs when the forward and reverse reactions (designated by a double arrow <-->) occurring simultaneously with products and reactants being constantly changing into each other. Understanding the nature of equilibrium allows researchers to control reaction conditions and maximize product yield. This is significant in industry, as you'll soon learn.
At equilibrium: (1) Forward and reverse reactions are occurring at equal rates, (2) There is no change in [reactants] and [products], (3) There is no change in macroscopic properties such as density and color, (4) The equilibrium can be approached from either the forward or reverse direction, (5), Equilibrium is dynamic, and (6) Any changes in reaction conditions can affect equilibrium.
Read about the reaction quotient (Q) here. It's also in your text. You need to know the relationship between Kc and Q.
It is important to understand the position of the equilibrium, which means determining whether reactants are favored or products are favored, and how to manipulate the position to maximize product yield and industry profitability. The law of chemical equilibrium states that at a given temperature, the ratio of the concentration of products (raised to the power of their molar coefficients) to the concentration of the reactants (also raised to the power of their molar coefficients) is a constant (see the below image). This constant is called the equilibrium constant, Kc. (Don't forget, this is on your quiz). Look at the example on page 182 and take a look at Bozeman Science's explanation of the concept.
Content Review:
Textbook: Chapter 7
Links: IB Chemistry Home Notes
Mission 1: Balance, Baby...Balance!
Mission Objectives: You should be able to:
1. Describe characteristics of chemical and physical systems at equilibrium.
2. Deduce the equilibrium constant (Kc) from an equation.
3. Explain Le Chatelier's Principle in your own words.
4. Define and explain reaction quotient.
5. Explain what it means when reactions must shift either to the left or the right.
6. Describe the effects of changing concentration, temperature and pressure on reactions at equilibrium.
7. Describe what happens when a catalyst is added to a reaction at equilibrium.
8. Explain what the Haber Process and the Contact Process are, and their significance.
Many chemical reactions are reversible and exist in a state of equilibrium. Equilibrium occurs when the forward and reverse reactions (designated by a double arrow <-->) occurring simultaneously with products and reactants being constantly changing into each other. Understanding the nature of equilibrium allows researchers to control reaction conditions and maximize product yield. This is significant in industry, as you'll soon learn.
At equilibrium: (1) Forward and reverse reactions are occurring at equal rates, (2) There is no change in [reactants] and [products], (3) There is no change in macroscopic properties such as density and color, (4) The equilibrium can be approached from either the forward or reverse direction, (5), Equilibrium is dynamic, and (6) Any changes in reaction conditions can affect equilibrium.
Read about the reaction quotient (Q) here. It's also in your text. You need to know the relationship between Kc and Q.
It is important to understand the position of the equilibrium, which means determining whether reactants are favored or products are favored, and how to manipulate the position to maximize product yield and industry profitability. The law of chemical equilibrium states that at a given temperature, the ratio of the concentration of products (raised to the power of their molar coefficients) to the concentration of the reactants (also raised to the power of their molar coefficients) is a constant (see the below image). This constant is called the equilibrium constant, Kc. (Don't forget, this is on your quiz). Look at the example on page 182 and take a look at Bozeman Science's explanation of the concept.
Page 183 shows you how to write equilibrium constant expressions. This is something you need to become very familiar with in the determination of Kc. Page 184 provides a summary table showing the relationships between reaction conditions and equilibrium position. You need to know this, period.
Le Chatelier's Principle is a rule that is useful for predicting the effect that changing conditions have on the equilibrium position. "If a change is made to a system in equilibrium, the balance between the forward and reverse reactions will shift to offset this change and return the system to equilibrium" (Oxford, 2014).
Watch the below videos to gain a better understanding of Le Chatelier's Principle.
Le Chatelier's Principle is a rule that is useful for predicting the effect that changing conditions have on the equilibrium position. "If a change is made to a system in equilibrium, the balance between the forward and reverse reactions will shift to offset this change and return the system to equilibrium" (Oxford, 2014).
Watch the below videos to gain a better understanding of Le Chatelier's Principle.
Factors that affect equilibrium:
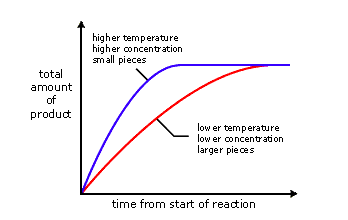
Change in concentration. When an increase in concentration of a reactant occurs and disrupts equilibrium, the rate of the forward reaction will increase. The reverse reaction does not change, so the rates are no longer equal. When equlibrium is re-established, the mixture will have new concentrations and the equlibrium will have shifted to the right to favor the products. Addition of reactant causes the system to respond by removing reactant to make more product, which favors the forward reaction.
Change in pressure. Equilibrium involving gases will be affected by a change in pressure if the reaction involves a change in the number of molecules. If a reaction at equilibrium is subject to an increase in pressure, the system responds by favoring the side with the smaller number of molecules. A decrease in pressure will cause a shift to the side with the larger number of molecules. As long as the temperature remains the same, Kc will be unchanged.
Change in temperature. Kc is temperature-sensitive, so it changes as temperature changes. Exothermic reactions (delta-H negative) release energy and endothermic reactions (delta-H positive) absorb energy. A negative delta-H means that the forward reaction is exothermic. If this reaction is subjected to a temperature decrease, the system responds by producing heat and favoring the forward reaction. Equilibrium shifts to to the right as a result. This means more product is formed. A new equilibrium is achieved and Kc changes.
Increasing the temperature favors the reverse reaction and shifts equilibrium to the left, decreasing the value of Kc and reducing the amount of product formed.
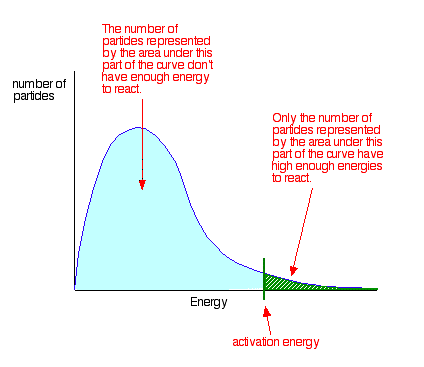
Adding catalysts. Catalysts speed up reactions by reducing Ea. The rate of both reactions will increase by the same factor, which has no effect on the equilibrium position. Adding a catalyst reduces the time it takes to achieve equilibrium.
Pages 188-189 provide excellent summary tables of the effects of temperature and catalysts on equilibrium. Study them.
The following video describes the Haber Process for ammonia production. You need to become familiar with this. The Haber Process is not listed in the SL chapter, but I'm going to insist that SL learns it anyway, as it makes problem solving a lot more useful if you know it.
Change in concentration. When an increase in concentration of a reactant occurs and disrupts equilibrium, the rate of the forward reaction will increase. The reverse reaction does not change, so the rates are no longer equal. When equlibrium is re-established, the mixture will have new concentrations and the equlibrium will have shifted to the right to favor the products. Addition of reactant causes the system to respond by removing reactant to make more product, which favors the forward reaction.
Change in pressure. Equilibrium involving gases will be affected by a change in pressure if the reaction involves a change in the number of molecules. If a reaction at equilibrium is subject to an increase in pressure, the system responds by favoring the side with the smaller number of molecules. A decrease in pressure will cause a shift to the side with the larger number of molecules. As long as the temperature remains the same, Kc will be unchanged.
Change in temperature. Kc is temperature-sensitive, so it changes as temperature changes. Exothermic reactions (delta-H negative) release energy and endothermic reactions (delta-H positive) absorb energy. A negative delta-H means that the forward reaction is exothermic. If this reaction is subjected to a temperature decrease, the system responds by producing heat and favoring the forward reaction. Equilibrium shifts to to the right as a result. This means more product is formed. A new equilibrium is achieved and Kc changes.
Increasing the temperature favors the reverse reaction and shifts equilibrium to the left, decreasing the value of Kc and reducing the amount of product formed.
Adding catalysts. Catalysts speed up reactions by reducing Ea. The rate of both reactions will increase by the same factor, which has no effect on the equilibrium position. Adding a catalyst reduces the time it takes to achieve equilibrium.
Pages 188-189 provide excellent summary tables of the effects of temperature and catalysts on equilibrium. Study them.
The following video describes the Haber Process for ammonia production. You need to become familiar with this. The Haber Process is not listed in the SL chapter, but I'm going to insist that SL learns it anyway, as it makes problem solving a lot more useful if you know it.
Here's a quick and easy tutorial on the Haber process. Below is the corresponding IB video by Richard Thornley, and he discusses both the Haber Process and the Contact Process, both which you need to know.
Describe the effects of adjusting the temperature and pressure on the formation of ammonia.
The Contact Process is the production of sulfuric acid. It is composed of three simple reactions: the combustion of sulfur to form sulfur dioxide, the oxidation of sulfur dioxide to sulfur trioxide, and the combination of sulfur trioxide with water to produce sulfuric acid. The overall rate depends on step 2; the oxidation of SO2 to SO3. Similar to the Haber process, one can predict the condition that will favor the formation of product.
The Contact Process is the production of sulfuric acid. It is composed of three simple reactions: the combustion of sulfur to form sulfur dioxide, the oxidation of sulfur dioxide to sulfur trioxide, and the combination of sulfur trioxide with water to produce sulfuric acid. The overall rate depends on step 2; the oxidation of SO2 to SO3. Similar to the Haber process, one can predict the condition that will favor the formation of product.
Let's practice!!!
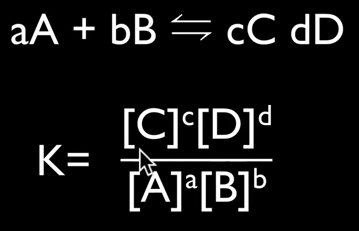

 RSS Feed
RSS Feed