Housekeeping: Need to see progress on IA topics and thinking this week. If you need to speak to me outside of class, I'm available M-W between 10:35 - 1:00.
Your bonding quiz (sections 4.1-4.3) is this Friday.
Agenda:
Intermolecular Forces
Metallic Bonding
Review
Content Review:
Links: Intermolecular Forces
Student Missions:
Mission 1: What's Keeping Us Together Other Than Love??
Mission Objectives. You should be able to...
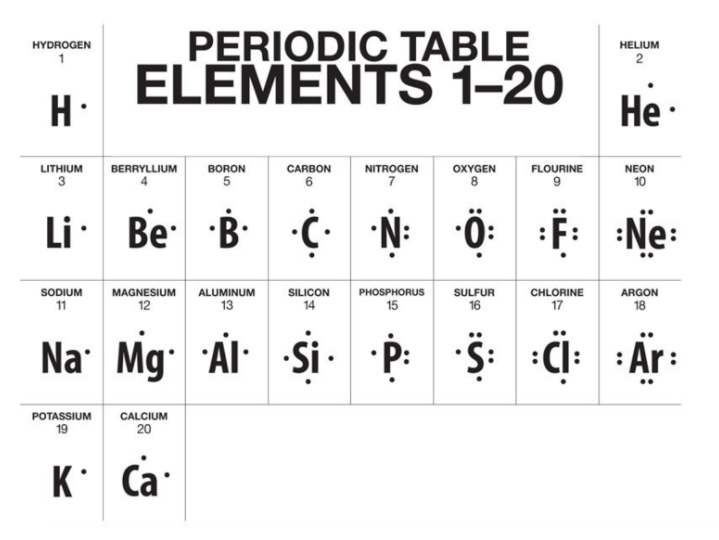
1. List and describe the types of intermolecular forces
2. Deduce the types of intermolecular forces present in substances based on their structure and chemical formulas.
3. Explain the relationship between physical properties of covalent compounds in terms of their structure and intermolecular forces.
Covalent bonds holds atoms together within molecules, but what forces hold molecules together? The answer depends on the polarity and size of the molecules involved, and so said intermolecular forces will vary. The strength of intermolecular forces determine the physical properties of a substance. Volatility, solubility, and conductivity can all be predicted and explained from knowledge of the nature of intermolecular forces.
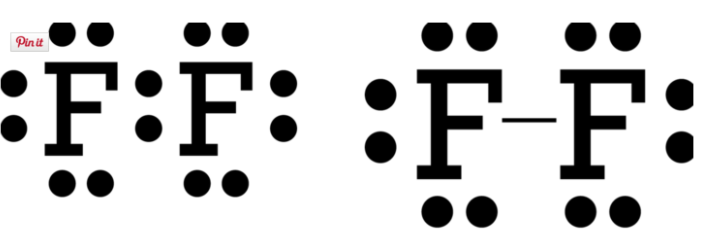
Dispersion forces (aka London forces): These are weak forces of attraction that occur between opposite ends of two temporary dipoles, usually with nonpolar molecules. A dipole occurs when the density of an electron cloud at any one moment be greater in one region of a molecule or atom. These dipoles are called temporary or instantaneous because they do not last long. When temporary dipoles influence the electron behavior of a nearby atom or molecule, induced dipoles result.
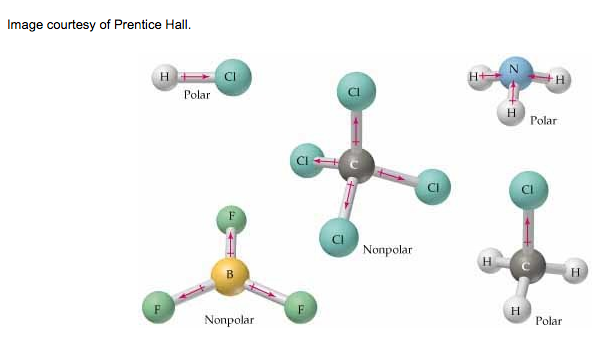
Polarizability is the ease of distortion of the electron cloud. Remember, most molecules that are asymmetric are polar. This means that they have a clear negative and positive end.
Factors that affect dispersion (London) forces:
1. number of valence electrons: greater the number of electrons, the larger the distance between valence electrons and the nucleus.
2. volume of electron cloud: large electron clouds can be polarized more easily.
3. molecule shape: large areas of interaction allow the London forces to have a greater magnitude, which leads to higher boiling points.
Dipole-dipole forces: Polar molecules that have a permanent separation of charge within their bonds as a result of electronegative differences have dipole-dipole attractions. Water has a clear positive end and a clear negative end. This is called a permanent dipole. When it bonds with another water molecule, a dipole-dipole attraction results. The strength varies depending on the distance and orientation of the dipoles.
The umbrella term van der Waals includes both dipole-dipole and dispersion forces.
Hydrogen bonding: When a molecule contains hydrogen covalently bonded to a very electronegative atom (fluorine, oxygen, or nitrogen), these molecules are attracted to each other by a particularly strong force called a hydrogen bond. These are specific instances of dipole-dipole attraction. The large electronegativity difference results in the electron pair being pulled away from hydrogen. It now exerts a strong attractive force on a lone pair of electrons in a nearby molecule.
Hydrogen bonds are the strongest intermolecular force. As a result, they cause the boiling points of substances to be higher than normal.
Your bonding quiz (sections 4.1-4.3) is this Friday.
Agenda:
Intermolecular Forces
Metallic Bonding
Review
Content Review:
Links: Intermolecular Forces
Student Missions:
Mission 1: What's Keeping Us Together Other Than Love??
Mission Objectives. You should be able to...
1. List and describe the types of intermolecular forces
2. Deduce the types of intermolecular forces present in substances based on their structure and chemical formulas.
3. Explain the relationship between physical properties of covalent compounds in terms of their structure and intermolecular forces.
Covalent bonds holds atoms together within molecules, but what forces hold molecules together? The answer depends on the polarity and size of the molecules involved, and so said intermolecular forces will vary. The strength of intermolecular forces determine the physical properties of a substance. Volatility, solubility, and conductivity can all be predicted and explained from knowledge of the nature of intermolecular forces.
Dispersion forces (aka London forces): These are weak forces of attraction that occur between opposite ends of two temporary dipoles, usually with nonpolar molecules. A dipole occurs when the density of an electron cloud at any one moment be greater in one region of a molecule or atom. These dipoles are called temporary or instantaneous because they do not last long. When temporary dipoles influence the electron behavior of a nearby atom or molecule, induced dipoles result.
Polarizability is the ease of distortion of the electron cloud. Remember, most molecules that are asymmetric are polar. This means that they have a clear negative and positive end.
Factors that affect dispersion (London) forces:
1. number of valence electrons: greater the number of electrons, the larger the distance between valence electrons and the nucleus.
2. volume of electron cloud: large electron clouds can be polarized more easily.
3. molecule shape: large areas of interaction allow the London forces to have a greater magnitude, which leads to higher boiling points.
Dipole-dipole forces: Polar molecules that have a permanent separation of charge within their bonds as a result of electronegative differences have dipole-dipole attractions. Water has a clear positive end and a clear negative end. This is called a permanent dipole. When it bonds with another water molecule, a dipole-dipole attraction results. The strength varies depending on the distance and orientation of the dipoles.
The umbrella term van der Waals includes both dipole-dipole and dispersion forces.
Hydrogen bonding: When a molecule contains hydrogen covalently bonded to a very electronegative atom (fluorine, oxygen, or nitrogen), these molecules are attracted to each other by a particularly strong force called a hydrogen bond. These are specific instances of dipole-dipole attraction. The large electronegativity difference results in the electron pair being pulled away from hydrogen. It now exerts a strong attractive force on a lone pair of electrons in a nearby molecule.
Hydrogen bonds are the strongest intermolecular force. As a result, they cause the boiling points of substances to be higher than normal.
Mission 2: Metallic Hook-Ups
Mission Objectives. You should be able to...
1. Explain the electrical conductivity and malleability in metals.
2. Explain the trends in melting points of metals.
Metallic bonding is the electrostatic attraction between a lattice of cations and delocalized electrons. Remember that delocalized electrons are not tied to a nucleus and free to move throughout the structure.
The strength of a metallic bond depends on three things: (1) number of valence electrons that can be delocalized, (2) charge of the metal ion, and (3) ionic radius of the metal cation.
Mission Objectives. You should be able to...
1. Explain the electrical conductivity and malleability in metals.
2. Explain the trends in melting points of metals.
Metallic bonding is the electrostatic attraction between a lattice of cations and delocalized electrons. Remember that delocalized electrons are not tied to a nucleus and free to move throughout the structure.
The strength of a metallic bond depends on three things: (1) number of valence electrons that can be delocalized, (2) charge of the metal ion, and (3) ionic radius of the metal cation.
Metals are good conductors of electricity because of the delocalized electrons. Charged species have to be free to move in order to conduct electricity. Metals are malleable, which means they can be hammered into shapes without breaking. Malleability is the result of cations sliding past each other, which leads to a rearrangement of the shape of the solid. The metallic bonds within the lattice structure do not have a defined direction; therefore, they are non-directional.
Melting points of metals depend on the strength of the attractive forces that hold the cations within the sea of delocalized electrons. The melting point of calcium is higher than potassium for the following reasons:
*Calcium has two delocalized electrons per atom and potassium has one. Therefore the electrostatic attraction between the cations and the delocalized electrons are greater.
**Calcium forms a 2+ cation and potassium forms a 1+. Therefore the electrostatic attraction between the positive ions and delocalized electrons is greater in calcium.
***Calcium has a smaller ionic radius that potassium. This implies that the delocalized electrons will be more strongly attracted to the Ca2+ ion.
Alloys, which are metallic solutions, can have a number of improved properties compared to their parent metallic element: increased strength, resistance to corrosion, enhanced magnetic properties, and greater ductility (ability to be drawn into wire). Adding additional atoms to the lattice reduces the ability of the cations to slide past each other and makes the metal stronger.
Melting points of metals depend on the strength of the attractive forces that hold the cations within the sea of delocalized electrons. The melting point of calcium is higher than potassium for the following reasons:
*Calcium has two delocalized electrons per atom and potassium has one. Therefore the electrostatic attraction between the cations and the delocalized electrons are greater.
**Calcium forms a 2+ cation and potassium forms a 1+. Therefore the electrostatic attraction between the positive ions and delocalized electrons is greater in calcium.
***Calcium has a smaller ionic radius that potassium. This implies that the delocalized electrons will be more strongly attracted to the Ca2+ ion.
Alloys, which are metallic solutions, can have a number of improved properties compared to their parent metallic element: increased strength, resistance to corrosion, enhanced magnetic properties, and greater ductility (ability to be drawn into wire). Adding additional atoms to the lattice reduces the ability of the cations to slide past each other and makes the metal stronger.
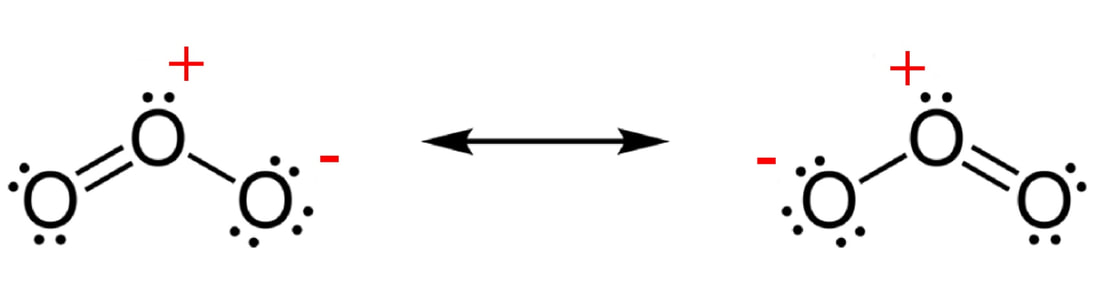
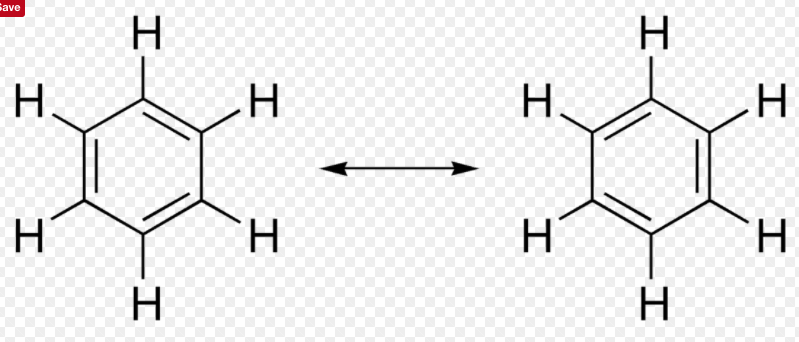

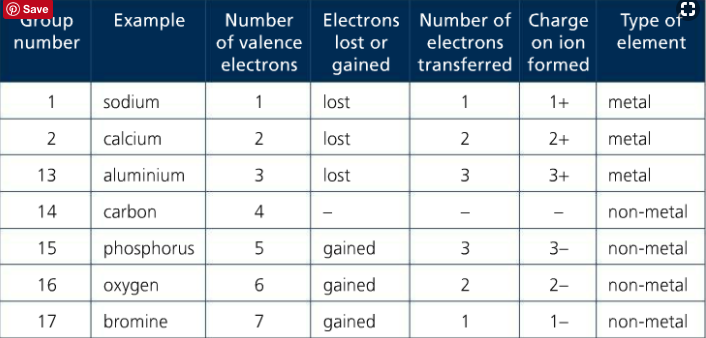
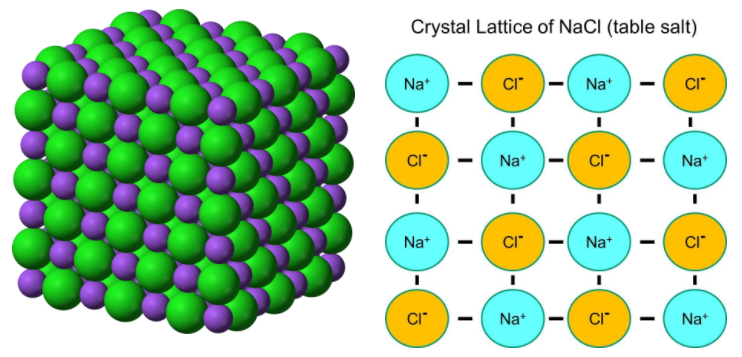

 RSS Feed
RSS Feed