Mission 1: Hess' Law
Mission Objectives. You should be able to...
1. Complete enthalpy calculations using Hess' Law.
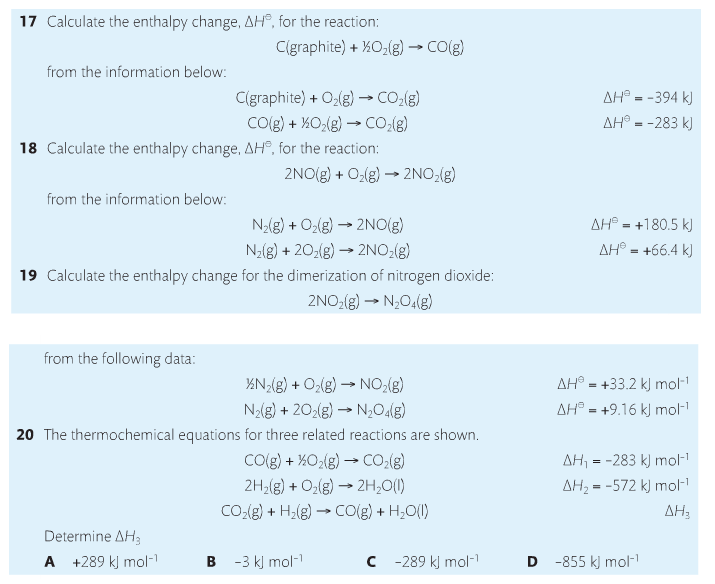
Hess' Law is an application of the conservation of energy law. A chemical equation shows the net reaction; it is a summary of a number of different reactions which are added together and result in an overall reaction. We will spend time working on questions in class.
Mission Objectives. You should be able to...
1. Complete enthalpy calculations using Hess' Law.
Hess' Law is an application of the conservation of energy law. A chemical equation shows the net reaction; it is a summary of a number of different reactions which are added together and result in an overall reaction. We will spend time working on questions in class.
Mission 2: Bond Enthalpy.
Mission Objectives. You should be able to...
1. Calculate the enthalpy changes from known bond enthalpy and comparison of these with experimentally measured values.
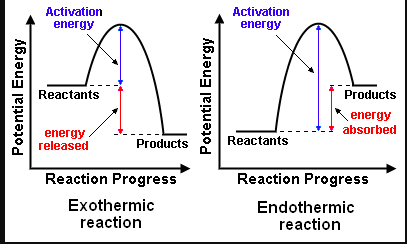
2. Sketch and evaluate potential energy profiles in determining whether reactants or products are more stable and if the reaction is endothermic or exothermic.
3. Discuss the bond strength in ozone relative to oxygen and its importance to the atmosphere.
Mr. Thornley is on the case. You will want to take notes from this video. He lays it out all clear and proper-like.
Mission Objectives. You should be able to...
1. Calculate the enthalpy changes from known bond enthalpy and comparison of these with experimentally measured values.
2. Sketch and evaluate potential energy profiles in determining whether reactants or products are more stable and if the reaction is endothermic or exothermic.
3. Discuss the bond strength in ozone relative to oxygen and its importance to the atmosphere.
Mr. Thornley is on the case. You will want to take notes from this video. He lays it out all clear and proper-like.
Image courtesy of www.socratic.org.
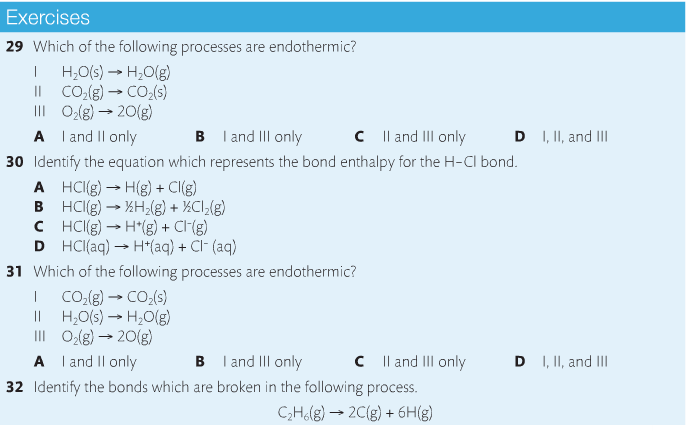
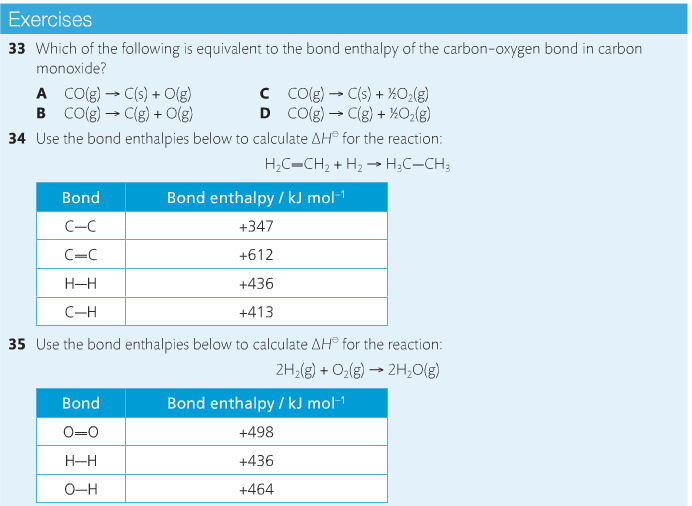
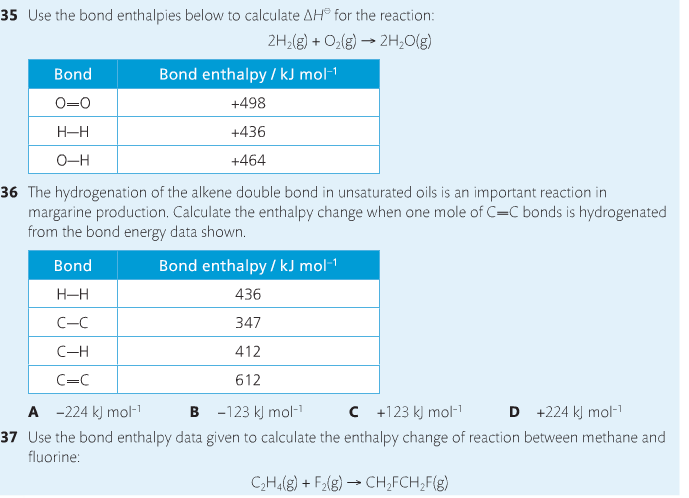
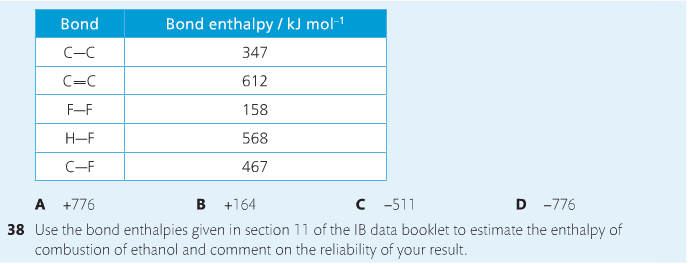
Bond breaking is an endothermic process. Energy must be absorbed to break the bonds between atoms and molecules. Bond making, by comparison, is an exothermic process. Energy is released when bonds are formed. Each bond type has its own energy requirements. Page 11 in your Data Booklet has a list of common bonds and their associated bond enthalpies. Recall that delta H for exothermic processes is negative and positive for endothermic processes. You need to be able to complete energy profiles for endothermic and exothermic reactions.
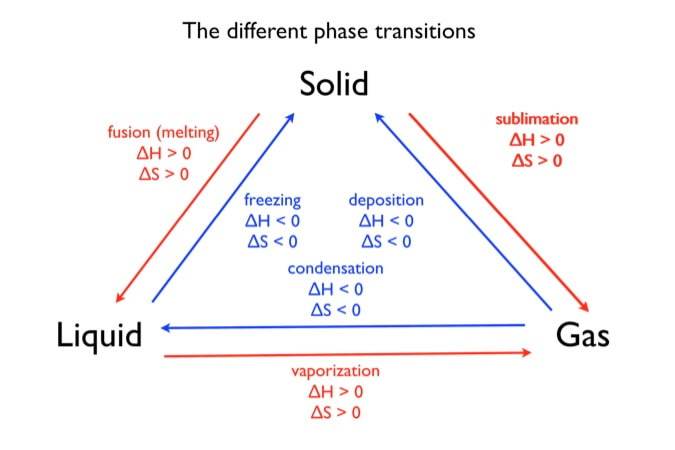
Phase changes that are endothermic: melting, boiling and sublimation. Phase changes that are exothermic: condensation, freezing and deposition. Read more about this here. When looking at thermochemical equations, take note of the phase changes as reactants turn into products. That will help you determine whether the reaction is endothermic or exothermic.
Phase changes that are endothermic: melting, boiling and sublimation. Phase changes that are exothermic: condensation, freezing and deposition. Read more about this here. When looking at thermochemical equations, take note of the phase changes as reactants turn into products. That will help you determine whether the reaction is endothermic or exothermic.
 RSS Feed
RSS Feed