Housekeeping: We are now in Chapter 03: Periodicity. This chapter is very short, so we can knock it out this week and take the exam over it and the atom within the next two weeks.
Agenda:
1. Discussion of the history of the periodic table
2. Periodic Trends activity
Content Review:
The Periodic Table Periodicity
Textbook:
Student Missions:
Mission 1: As Usual, HIStory.
Mission Objectives: You should be able to...
1. Track the development of the periodic table from Antoine Lavoisier to Henry Moseley.
2. Explain why Mendeleev's work was so significant.
The PT has a long and sordid (I wish) history and is the result of the work of many scientists. Dmitri Mendeleev gets the most credit because he found an organizational system that worked better than others and could make predictions about future elements. The Royal Society of Chemistry provides an in-depth review which you should read outside of class. However, Mendeleev's version of the PT didn't quite work (which element was the one to cause him problems?) because he ordered the elements by increasing atomic mass. We now know, thanks to Henry Moseley, that elements are ordered by increasing atomic number.
Agenda:
1. Discussion of the history of the periodic table
2. Periodic Trends activity
Content Review:
The Periodic Table Periodicity
Textbook:
Student Missions:
Mission 1: As Usual, HIStory.
Mission Objectives: You should be able to...
1. Track the development of the periodic table from Antoine Lavoisier to Henry Moseley.
2. Explain why Mendeleev's work was so significant.
The PT has a long and sordid (I wish) history and is the result of the work of many scientists. Dmitri Mendeleev gets the most credit because he found an organizational system that worked better than others and could make predictions about future elements. The Royal Society of Chemistry provides an in-depth review which you should read outside of class. However, Mendeleev's version of the PT didn't quite work (which element was the one to cause him problems?) because he ordered the elements by increasing atomic mass. We now know, thanks to Henry Moseley, that elements are ordered by increasing atomic number.
Mission 2: It's PERIODIC Because...
Mission Objective. You should be able to...
1. Define, describe and explain periodic trends across periods and down groups.
...because the organization of elements via atomic number showed properties (with a magical number of 8, of course) that were...wait for it...periodic! Elements in the same columns tend to behave the same way. We now know this is because of their valence electrons. Because of periodicity, trends in elements can be predicted. We discuss four types of trends: ionization energy, electronegativity, atomic radius and ionic radius.
Mission Objective. You should be able to...
1. Define, describe and explain periodic trends across periods and down groups.
...because the organization of elements via atomic number showed properties (with a magical number of 8, of course) that were...wait for it...periodic! Elements in the same columns tend to behave the same way. We now know this is because of their valence electrons. Because of periodicity, trends in elements can be predicted. We discuss four types of trends: ionization energy, electronegativity, atomic radius and ionic radius.
You can review these basic trends on the periodicity webpage. Be sure you know what each one represents.
Your textbook goes into some detail about trends in electron affinity, metallic/nonmetallic character, and properties of metal and nonmetal oxides.
Electron affinity is defined as the energy required to detach an electron from the single charged negative ion in the gas phase. This process is exothermic. (Ionization energy is endothermic) The more negative the electron affinity value, the greater the attraction of the ion for the electron. You will need to reference the data booklet to see the values for the elements.
Metallic character decreases across a period and increases down a group. It follows the same trend as atomic radius. Metals have low IEs and tend to lose electrons. Therefore they can be oxidized. Nonmetals have high IEs and tend to gain electrons. Therefore they can be reduced.
Metal oxides are basic; they react with water to form metal hydroxides (see p. 86). Nonmetal oxides are acidic; they react with water to form acid solutions.
Your textbook goes into some detail about trends in electron affinity, metallic/nonmetallic character, and properties of metal and nonmetal oxides.
Electron affinity is defined as the energy required to detach an electron from the single charged negative ion in the gas phase. This process is exothermic. (Ionization energy is endothermic) The more negative the electron affinity value, the greater the attraction of the ion for the electron. You will need to reference the data booklet to see the values for the elements.
Metallic character decreases across a period and increases down a group. It follows the same trend as atomic radius. Metals have low IEs and tend to lose electrons. Therefore they can be oxidized. Nonmetals have high IEs and tend to gain electrons. Therefore they can be reduced.
Metal oxides are basic; they react with water to form metal hydroxides (see p. 86). Nonmetal oxides are acidic; they react with water to form acid solutions.
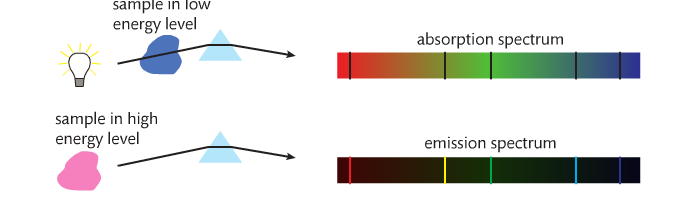
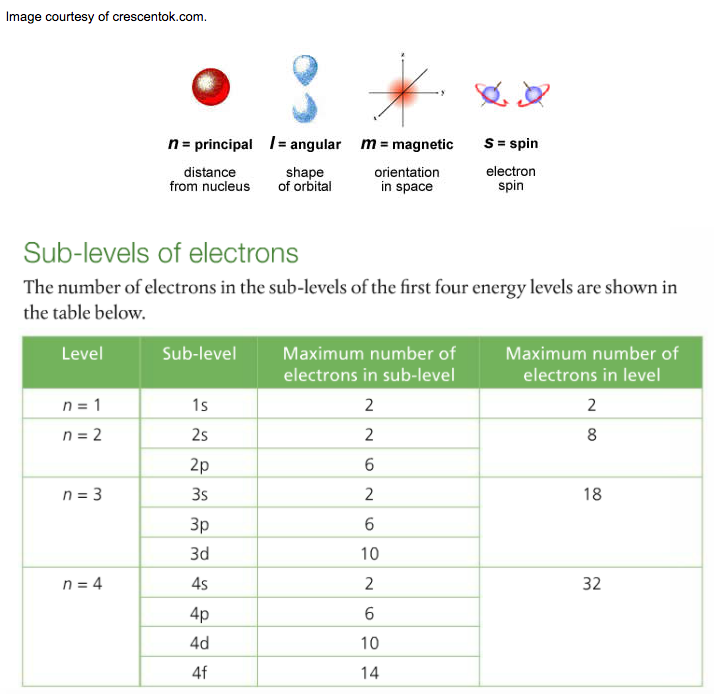
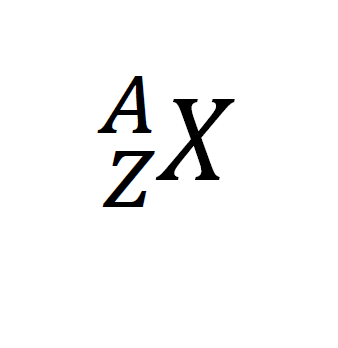
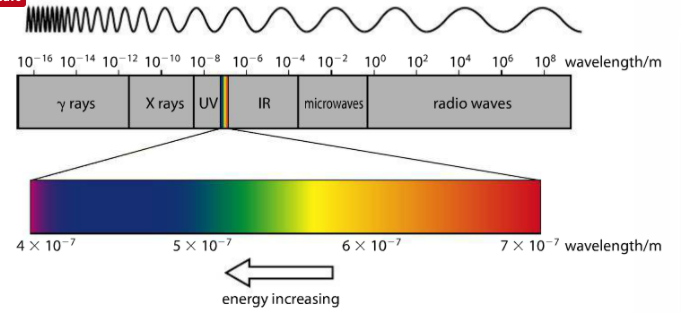
 RSS Feed
RSS Feed