Periodic Trends
Periodicity Powerpoint
Atomic Radius (AR)
As atoms gain protons, their sizes decrease because of the increased positive pull of the nucleus on the valence electrons. Think in terms of a game of tug of war; with the nucleus on one end and the valence electrons on the other. Adding more protons pulls the valence electrons closer. Atomic number increases from left to right, so atoms decrease in size from left to right. Atomic number increases from top to bottom, so atoms increase in size from top to bottom.
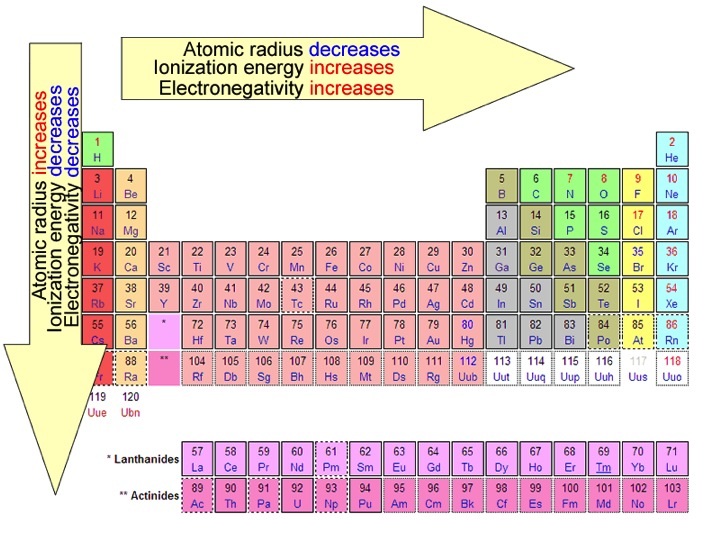
Therefore, atomic radii decrease across a period and increase down a group.
Ionic Radius (IR)
Metals form cations easily, and nonmetals form anions easily. Cations are always smaller than the neutral atoms from which they form, because the stripping of the valence electrons increases the attraction from the nucleus. As a result, the nucleus pulls the remaining electrons closer. Anions are always larger than the neutral atoms from which they form, because adding electrons decreases the nuclear pull.
Therefore, just like atomic radii, ionic radii decrease across a period and increase down a group.
Ionization Energy (IE)
When an atom gains or loses an electron, it becomes an ion. Positively charged ions are called cations and negatively charged ions are called anions. The energy required to overcome the attraction of the nuclear charge and remove an electron is called ionization energy.
Removing one electron results in the formation of a cation with a +1 charge (and the energy required to do so is called the first ionization energy). Removing two electrons (which is more difficult) results in a cation with a +2 charge (second ionization energy). As the ionization energy increases, it becomes more and more difficult to remove electrons. Larger atoms' valence electrons are further away from the nucleus, and therefore have lower IEs.
Similarly, adding electrons increases the negative charge. Adding one electron results in the formation of an anion with a -1 charge. Adding two electrons results in an anion with a -2 charge, and so on and so forth.
Therefore, ionization energy increases across a period and decreases down a group.
Electronegativity (EN)
The electronegativity of an element is the tendency of its atoms to attract electrons when they are chemically combined with another element. Noble gases do not have electronegativity values because they do not readily form compounds; they are inert. Electronegativity follows the same pattern as ionization energy and the opposite pattern of atomic radius.
Therefore, ionization energy increases across a period and decreases down a group.
This summary image is courtesy of WilenskyChemistry:
As atoms gain protons, their sizes decrease because of the increased positive pull of the nucleus on the valence electrons. Think in terms of a game of tug of war; with the nucleus on one end and the valence electrons on the other. Adding more protons pulls the valence electrons closer. Atomic number increases from left to right, so atoms decrease in size from left to right. Atomic number increases from top to bottom, so atoms increase in size from top to bottom.
Therefore, atomic radii decrease across a period and increase down a group.
Ionic Radius (IR)
Metals form cations easily, and nonmetals form anions easily. Cations are always smaller than the neutral atoms from which they form, because the stripping of the valence electrons increases the attraction from the nucleus. As a result, the nucleus pulls the remaining electrons closer. Anions are always larger than the neutral atoms from which they form, because adding electrons decreases the nuclear pull.
Therefore, just like atomic radii, ionic radii decrease across a period and increase down a group.
Ionization Energy (IE)
When an atom gains or loses an electron, it becomes an ion. Positively charged ions are called cations and negatively charged ions are called anions. The energy required to overcome the attraction of the nuclear charge and remove an electron is called ionization energy.
Removing one electron results in the formation of a cation with a +1 charge (and the energy required to do so is called the first ionization energy). Removing two electrons (which is more difficult) results in a cation with a +2 charge (second ionization energy). As the ionization energy increases, it becomes more and more difficult to remove electrons. Larger atoms' valence electrons are further away from the nucleus, and therefore have lower IEs.
Similarly, adding electrons increases the negative charge. Adding one electron results in the formation of an anion with a -1 charge. Adding two electrons results in an anion with a -2 charge, and so on and so forth.
Therefore, ionization energy increases across a period and decreases down a group.
Electronegativity (EN)
The electronegativity of an element is the tendency of its atoms to attract electrons when they are chemically combined with another element. Noble gases do not have electronegativity values because they do not readily form compounds; they are inert. Electronegativity follows the same pattern as ionization energy and the opposite pattern of atomic radius.
Therefore, ionization energy increases across a period and decreases down a group.
This summary image is courtesy of WilenskyChemistry: