Electron Behavior
When drawing orbital diagrams and writing electron configurations, there are a few rules one must follow:
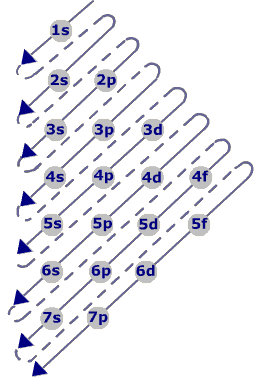
1. Aufbau Diagram: This is the sequence in which orbitals are filled. Electrons fill orbitals at the lowest available energy state before filling higher states. So 2p cannot be filled before 2s is filled, and 2s can't be filled before 1s is filled.
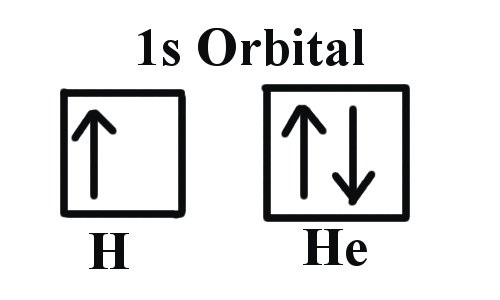
2. Pauli Exclusion Principle: The maximum number of electrons that can occupy an orbital is two, spinning in opposite directions.
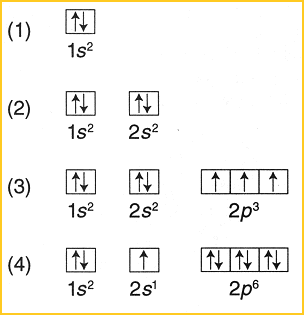
3. Hund's Rule: Every orbital must have one electron before any can have two electrons. This is the case for p, d, & f sublevels.
Content Source: Mr. Kent's Chemistry Page
Image Sources: CIR Rm 6 (Hund's Rule), ChemWiki (P.E.P.), & ChemGuide (Aufbau )
Educreate lessons:
Orbitals
Aufbau Diagrams
Pauli Exclusion Principle
Hund's Rule
Click here to learn how to draw orbital diagrams and write electron configurations.
Quantum Theory Notes
Orbital Diagrams
Image Sources: CIR Rm 6 (Hund's Rule), ChemWiki (P.E.P.), & ChemGuide (Aufbau )
Educreate lessons:
Orbitals
Aufbau Diagrams
Pauli Exclusion Principle
Hund's Rule
Click here to learn how to draw orbital diagrams and write electron configurations.
Quantum Theory Notes
Orbital Diagrams