Housekeeping: I want to check your understanding of chemical bonding. So take out the practice problems from last week and we will go through them. Then we will review for your quiz. If you are still struggling with nomenclature and formula writing, now is the time to speak up.
Agenda:
1. Chemical bonding review
2. VSEPR Theory
Content Review:
Text: Chapters 10 & 11
Links: Chemical Equations The Mole
Student Missions:
Mission 1: Getting in Shape!
Mission Objectives: You should be able to...
1. Determine the structure of simple molecules using VSEPR Theory.
2. Predict the molecular geometry for simple compounds.
3. List and describe the three kinds of intermolecular forces.
I already have these lessons prepared for another class. Click on the links.
VSEPR Theory
Intermolecular Forces
More on intermolecular forces: Chemical bonds are stronger than intermolecular forces and more energy is required for their formation.
Temporary dipoles occurs when electrons are dense on one end of a molecule. All molecules can become temporary dipoles. Permanent dipoles are molecules which have a clear negative and positive end (two poles) resulting from polar bonds.
Water molecules have high intermolecular forces resulting from hydrogen bonding, which is the strongest IMF.
IMFs can determine boiling points and states of matter. Read about it here and here.
Mission 2: The Five Families.
Mission Objective. You should be able to...
1. Identify the five kinds of chemical equations
2. Balance chemical equations
I don't feel the need to get redundant when we can just go here. It is expected that you will be able to identify/classify any equation that is given to you based on what the reactants look like. So pay attention!
Download this handout and practice writing equations. You are turning formulas into words, which we have been doing since last week.
Mission 3: Count 'em Up!!
Mission Objective. You should be able to...
1. Balance chemical equations.
2. Define and understand the significance of "delta" in a chemical equation.
The basic rule about balancing equations is this: The number of atoms on the reactant side should equal the number of atoms on the product side. The easiest way to do this is to identify individual atoms and count. Add coefficients to the beginning of chemical formulas to make the math work. Coefficients go AT THE BEGINNING of a formula; nowhere else. For polyatomic ions, if they appear on both sides of the equation, you can keep them together as a unit. It makes it easier to count that way.
In chemical equations, a delta (triangle) is used over the arrow to denote that heat is used so that the reaction can take place. You are expected to know this.
Here is an adorable conceptual video to help you understand how and why equations must be balanced. Hint: we cannot go around breaking laws and stuff! The second equation gives you five simple rules for balancing equations.
Agenda:
1. Chemical bonding review
2. VSEPR Theory
Content Review:
Text: Chapters 10 & 11
Links: Chemical Equations The Mole
Student Missions:
Mission 1: Getting in Shape!
Mission Objectives: You should be able to...
1. Determine the structure of simple molecules using VSEPR Theory.
2. Predict the molecular geometry for simple compounds.
3. List and describe the three kinds of intermolecular forces.
I already have these lessons prepared for another class. Click on the links.
VSEPR Theory
Intermolecular Forces
More on intermolecular forces: Chemical bonds are stronger than intermolecular forces and more energy is required for their formation.
Temporary dipoles occurs when electrons are dense on one end of a molecule. All molecules can become temporary dipoles. Permanent dipoles are molecules which have a clear negative and positive end (two poles) resulting from polar bonds.
Water molecules have high intermolecular forces resulting from hydrogen bonding, which is the strongest IMF.
IMFs can determine boiling points and states of matter. Read about it here and here.
Mission 2: The Five Families.
Mission Objective. You should be able to...
1. Identify the five kinds of chemical equations
2. Balance chemical equations
I don't feel the need to get redundant when we can just go here. It is expected that you will be able to identify/classify any equation that is given to you based on what the reactants look like. So pay attention!
Download this handout and practice writing equations. You are turning formulas into words, which we have been doing since last week.
Mission 3: Count 'em Up!!
Mission Objective. You should be able to...
1. Balance chemical equations.
2. Define and understand the significance of "delta" in a chemical equation.
The basic rule about balancing equations is this: The number of atoms on the reactant side should equal the number of atoms on the product side. The easiest way to do this is to identify individual atoms and count. Add coefficients to the beginning of chemical formulas to make the math work. Coefficients go AT THE BEGINNING of a formula; nowhere else. For polyatomic ions, if they appear on both sides of the equation, you can keep them together as a unit. It makes it easier to count that way.
In chemical equations, a delta (triangle) is used over the arrow to denote that heat is used so that the reaction can take place. You are expected to know this.
Here is an adorable conceptual video to help you understand how and why equations must be balanced. Hint: we cannot go around breaking laws and stuff! The second equation gives you five simple rules for balancing equations.
Mission 4: The Mole.
Mission Objectives. You should be able to...
1. Define the mole
2. Complete mole conversions using mass and molar mass.
Mission Objectives. You should be able to...
1. Define the mole
2. Complete mole conversions using mass and molar mass.
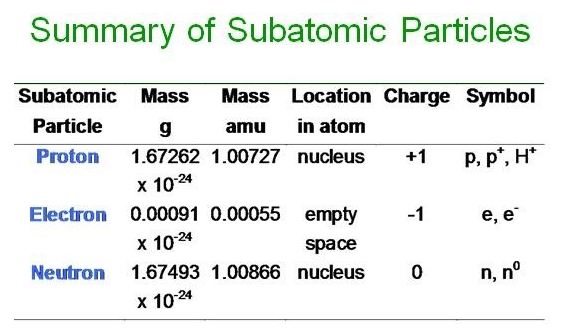
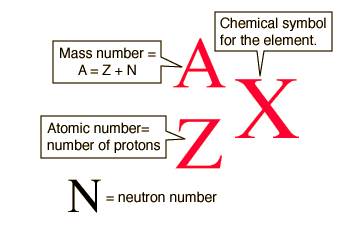
The mole is the SI unit for counting particles. One mole of any substance contains 6.02 * 10^23 particles. This number is known as Avogadro's Number, named after Italian scientist Amedeo Avogadro.
"Particles" is a generic term for atoms, formula units, and molecules. Avogadro's number of atoms clearly references an element, Avogadro's number of formula units references an ionic compound, and Avogadro's number of molecules references a covalent compound.
The molar mass of a substance is either the element's atomic mass on the periodic table, or the sum of the atomic masses of a formula unit or a compound.
For example: Hydrogen's molar mass is 1.01 g/mol. But water, which is H2O, is 18.02 g/mol. The 18.02 comes from adding the two hydrogens (2.02) with oxygen (rounded up to 16.00). So whenever you are dealing with the molar mass of a compound, you must add up the atomic mass of all of the atoms in that compound.
Sample mole problems:
1) How many moles are in 25 grams of water?
2) How many grams are in 4.5 moles of Li2O?
3) How many molecules are in 23 moles of oxygen?
4) How many moles are in 3.4 x 10^23 molecules of H2SO4?
5) How many molecules are in 25 grams of NH3?
6) How many grams are in 8.2 x 10^22 molecules of N2I6?
"Particles" is a generic term for atoms, formula units, and molecules. Avogadro's number of atoms clearly references an element, Avogadro's number of formula units references an ionic compound, and Avogadro's number of molecules references a covalent compound.
The molar mass of a substance is either the element's atomic mass on the periodic table, or the sum of the atomic masses of a formula unit or a compound.
For example: Hydrogen's molar mass is 1.01 g/mol. But water, which is H2O, is 18.02 g/mol. The 18.02 comes from adding the two hydrogens (2.02) with oxygen (rounded up to 16.00). So whenever you are dealing with the molar mass of a compound, you must add up the atomic mass of all of the atoms in that compound.
Sample mole problems:
1) How many moles are in 25 grams of water?
2) How many grams are in 4.5 moles of Li2O?
3) How many molecules are in 23 moles of oxygen?
4) How many moles are in 3.4 x 10^23 molecules of H2SO4?
5) How many molecules are in 25 grams of NH3?
6) How many grams are in 8.2 x 10^22 molecules of N2I6?
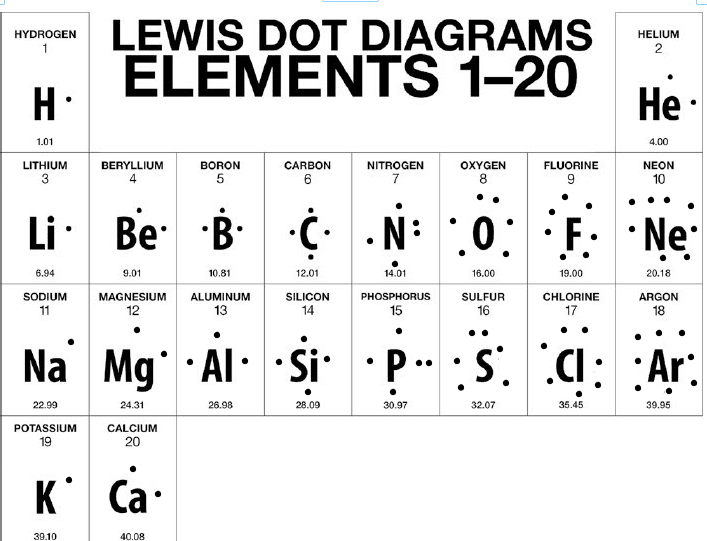
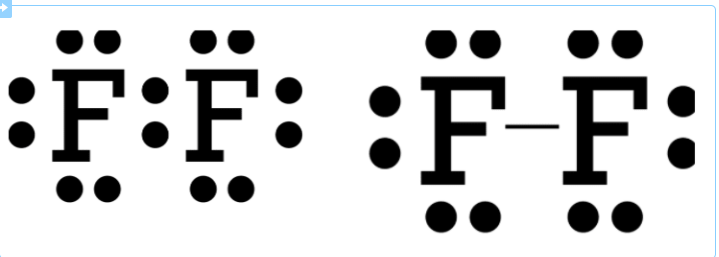
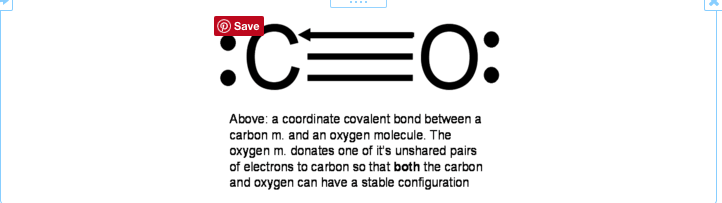
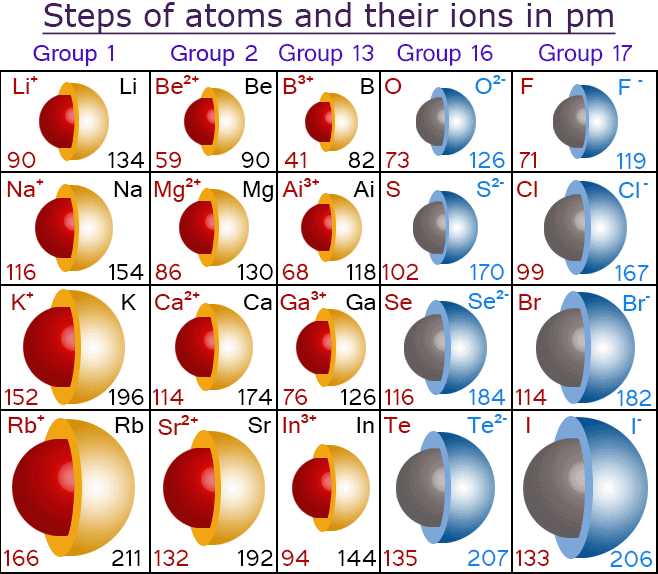
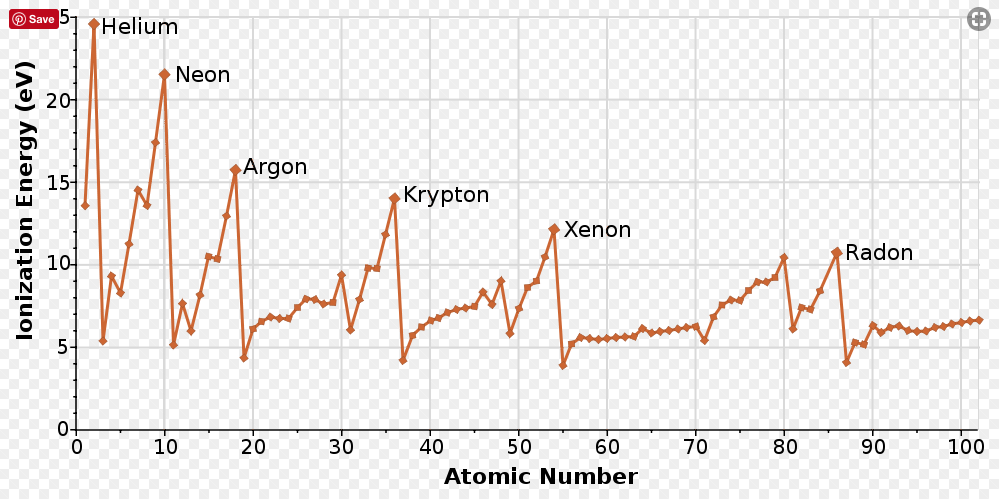
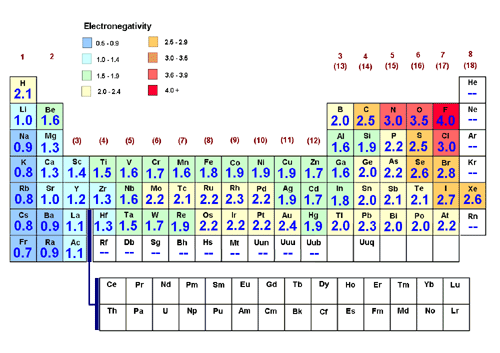
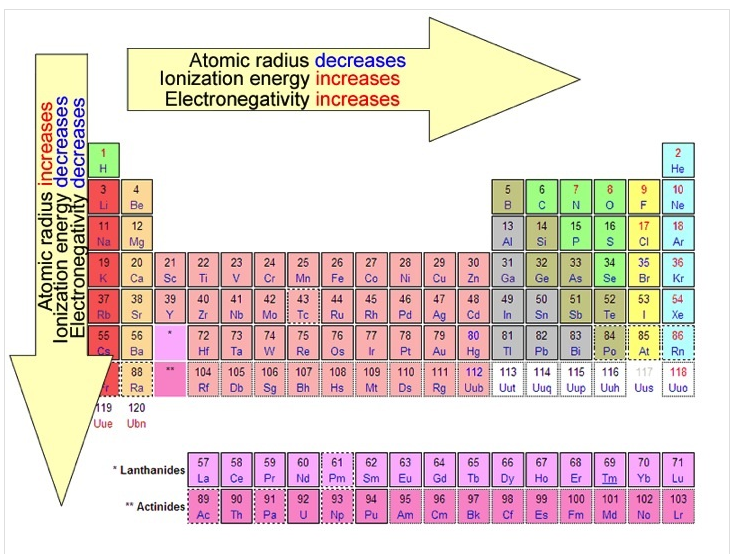
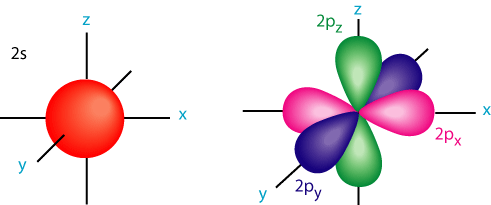
 RSS Feed
RSS Feed