Housekeeping: We are now studying waves. You will be given a "textbook" that contains your readings and whatever practice problems you'll need.
Content Review: BBC Bitesize colorado.edu
Student Missions:
Mission 1: Wave Properties
Mission Objectives: You should be able to...
1. Explain how waves transfer energy without transferring matter.
2. Compare & contrast longitudinal and transverse waves.
3. Relate wave speed, wavelength & frequency
Content Review: BBC Bitesize colorado.edu
Student Missions:
Mission 1: Wave Properties
Mission Objectives: You should be able to...
1. Explain how waves transfer energy without transferring matter.
2. Compare & contrast longitudinal and transverse waves.
3. Relate wave speed, wavelength & frequency
First of all, watch the above video. Secondly, read Section 14.1 in your textbook. It covers wave basics. You are expected to know the vocabulary. You will investigate waves using springs using the design lab on page 330. You will take measurements on amplitude, wavelength and frequency. You need to be able to calculate the speed of the wave you generate (page 333 shows you how to do this) with the spring.
You will eventually submit a research question, hypothesis, data, analysis and conclusion, but not today. You cannot use the RQ and hypothesis listed on page 330. Once you are done with the activity, begin working the problems on page 335.
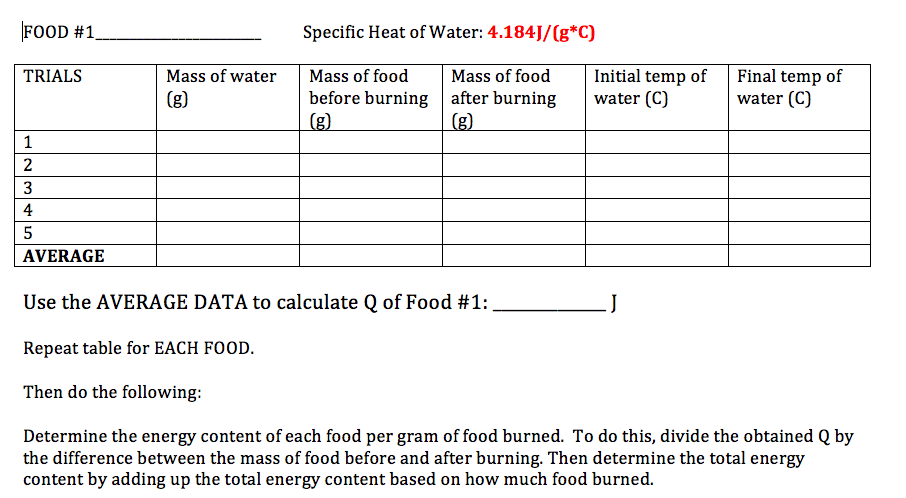
Use the below to help you with organizing your data and complete calculations.
You will eventually submit a research question, hypothesis, data, analysis and conclusion, but not today. You cannot use the RQ and hypothesis listed on page 330. Once you are done with the activity, begin working the problems on page 335.
Use the below to help you with organizing your data and complete calculations.
| wave_data.docx |
Play around with waves using this simulator. Or this one.
Mission 2: Wave Behavior
Mission Objectives: You should be able to...
1. Relate a wave's speed to the medium in which it travels.
2. Explain the concept of interference.
3. Describe how waves are reflected and refracted at boundaries between media and explain how waves diffract.
4. Apply the principle of superposition to the phenomenon of interference.
5. Predict the shape of a wave formed by interference.
Section 14.2 covers wave behavior. Make sure you read it and get all the vocabulary down.
Mission 2: Wave Behavior
Mission Objectives: You should be able to...
1. Relate a wave's speed to the medium in which it travels.
2. Explain the concept of interference.
3. Describe how waves are reflected and refracted at boundaries between media and explain how waves diffract.
4. Apply the principle of superposition to the phenomenon of interference.
5. Predict the shape of a wave formed by interference.
Section 14.2 covers wave behavior. Make sure you read it and get all the vocabulary down.
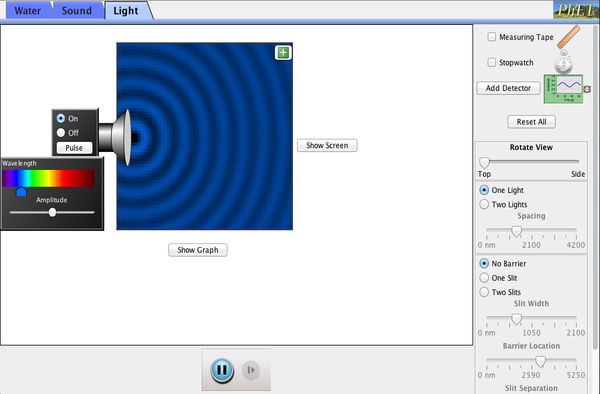
Below is a pHet simulation on wave interference. Play around with it. Below that is another powerpoint that goes into detail about refraction, reflection, interference and diffraction.
Mission 3: Something Snells Up in Here!
Mission Objectives. You should be able to...
1. Explain Snell's Law.
2. Solve problems using Snell's Law.
Mission Objectives. You should be able to...
1. Explain Snell's Law.
2. Solve problems using Snell's Law.
Let's practicesolving Snell's Law problems.

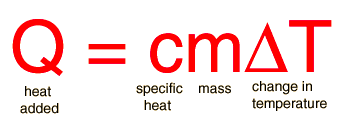
 RSS Feed
RSS Feed