You also owe me two labs: the macromolecule investigation and the separating mixtures lab. The macromolecules lab is due tomorrow (you should have been done with this last week). The separating mixtures lab will be due Friday.
You will finish the separating mixtures lab today in class and then we will go over what you will submit.
Agenda:
1. Housekeeping
2. Finish the lab
3. Discussion of write up/submissions
4. Atomic Theory & Structure (I)
Content Review:
Weebly Links: Matter
Weebly Links: The Atom Atomic Theory Nuclear Chemistry Electrons
Link: Substances & Mixtures Elements & Compounds Matter, Elements & Atoms
Student Missions:
Mission 1: Itty Bitty Things...Rack 'Em Up!!!
Mission Objectives. You should be able to...
1. Describe the structure of the atom.
2. Explain how atoms differ.
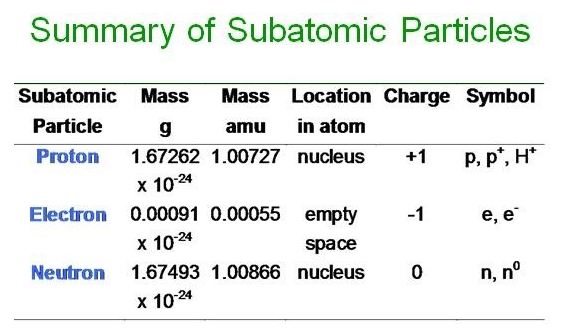
All neutral atoms contain the same number of protons & electrons. The number of protons determines the element's identity. For instance, 8 protons = oxygen. 17 protons = chlorine. 20 protons = calcium. This does not change.
Electrons determine chemical behavior. Valence electrons (electrons in the outermost energy levels) are significant in this regard, because the number of valence electrons determine how an element behaves in certain conditions. Elements with an octet (8 valence electrons) are unusually stable and do not combine to form compounds (noble gases have an octet, with the exception of helium).
Neutrons determine isotopes. They do not affect the charge or the element's identity. However, they do affect the mass of the nucleus. Several elements have multiple isotopes.
Mission Objectives. You should be able to...
1. Calculate the atomic mass of an element.
2. Identify isotopes of various elements.
Atoms with different numbers of neutrons are called isotopes. For instance, carbon has fifteen known isotopes, but only three are commonly referenced: carbon-12, carbon-13, and carbon-14.
All three isotopes have six protons (carbon's atomic number), but C-12 has 6 neutrons, C-13 has 7 neutrons, and C-14 has 8 neutrons.
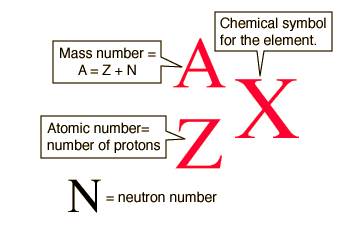
Isotopes are usually written in a form called standard notation, which includes the mass number (A), the atomic number (Z) and the symbol of the element (X).
Well, that's because the average atomic mass is the average of all isotopes for any one element. In order to calculate the AAM, you need to know the percent abundance of each isotope (this should be a percentage, which you turn into a decimal), and the atomic mass of each isotope. DO NOT ROUND THESE NUMBERS. You will do your rounding at the very end.
Multiply the percent abundance (now a decimal) by its atomic mass. Do this for each isotope. Then add all the isotopes together. Your answer should equal the value that is listed on the periodic table.
ChemWiki went hard by demonstrating how to calculate AAM with pictures.
Wanna practice? Of course you do!
Here's a short video showing how it's done.
We are going to do the Candium Lab, where you determine the average atomic mass of a bag of candy. This lab is your first write-up, and I've included the template for you to follow. It is important that you follow the instructions and ask questions when you aren't sure whether something should be included. You'll need a calculator.
Mission 3: Atomic Theory. How did we go from "nothing" to "something" to "something really complex?"
Mission Objectives. You should be able to...
1. Trace the development of atomic theory from ancient times to now.
2. Explain why models have to be revised and refined.
3. Describe how our understanding of the atom has changed over time.
What we know about the atom has changed over time because of technological advances, obviously. The book does not provide enough historical context, so you can add to your knowledge base by reading up on atomic theory. Ask yourself, "How is the ability to understand a fact of nature is limited by our ability to observe it?"
You will track the development and refinement of atomic theory and resultant models from Democritus to Bohr. I will put you into groups and assign you a theorist/model. There will be pictures required. You will present your research on _________________.
Regardless of your assigned model, be able to answer this question: Why is the Bohr model still used as a reference even though it is no longer the accepted model? What is the currently accepted model?
Some links to help you are below.
1. BBC Bitesize
2. Modern Atomic Theory
3. Early Atomic Theory
4. Atomic Theory Timeline
5. The Quantum Mechanical Model
Questions you should be able to answer:
1. Who is your scientist/theorist and what were their scientific qualifications? No more than 3-4 sentences explaining this.
2. What specific particle did they study, or did they study the atom in general?
3. What kinds of experiments did they do? Describe in detail.
4. What was the name of their atomic model?
5. Provide a picture of the model.
6. Why did the model eventually have to be replaced, if it was replaced? In other words, models change because they can no longer sufficiently explain certain phenomena. Why did your assigned atomic model have to be replaced?
Homework: Begin working on your presentations. Grading Rubric.
 RSS Feed
RSS Feed