Housekeeping: Looking at the rest of this month, here's what I have scheduled:
This week, periodicity. Next week, there needs to be an exam over atomic structure and periodicity. The best (and only) date to do it is September 20th. The week after that, you guys will do a Meet the Elements lab on September 29, 2017.
The cereal box/cookie box is due on Tuesday, September 19.
Agenda:
1. Project Update
2. Electron Behavior
3. Discussion and analysis of periodic trends
Content Review:
Links: The Periodic Table Periodic Trends
Textbook Readings: Chapter 6.
Student Missions:
Mission 1: What We Already Know.
Mission Objectives. You should be able to...
1. Explain how Mendeleev organized the first periodic table.
2. Describe the ways in which the periodic table is organized.
3. Name the families of the periodic table.
The PT has a long and sordid (I wish) history and is the result of the work of many scientists. Dmitri Mendeleev gets the most credit because he found an organizational system that worked better than others and could make predictions about future elements. The Royal Society of Chemistry provides an in-depth review which you should read outside of class. However, Mendeleev's version of the PT didn't quite work (which element was the one to cause him problems?) because he ordered the elements by increasing atomic mass. We now know, thanks to Henry Moseley, that elements are ordered by increasing atomic number.
This week, periodicity. Next week, there needs to be an exam over atomic structure and periodicity. The best (and only) date to do it is September 20th. The week after that, you guys will do a Meet the Elements lab on September 29, 2017.
The cereal box/cookie box is due on Tuesday, September 19.
Agenda:
1. Project Update
2. Electron Behavior
3. Discussion and analysis of periodic trends
Content Review:
Links: The Periodic Table Periodic Trends
Textbook Readings: Chapter 6.
Student Missions:
Mission 1: What We Already Know.
Mission Objectives. You should be able to...
1. Explain how Mendeleev organized the first periodic table.
2. Describe the ways in which the periodic table is organized.
3. Name the families of the periodic table.
The PT has a long and sordid (I wish) history and is the result of the work of many scientists. Dmitri Mendeleev gets the most credit because he found an organizational system that worked better than others and could make predictions about future elements. The Royal Society of Chemistry provides an in-depth review which you should read outside of class. However, Mendeleev's version of the PT didn't quite work (which element was the one to cause him problems?) because he ordered the elements by increasing atomic mass. We now know, thanks to Henry Moseley, that elements are ordered by increasing atomic number.
Mission 2: It's PERIODIC Because...
Mission Objective. You should be able to...
1. Explain the relationships between atomic number and ionization energy, atomic radius, electronegativity, and ionic radii.
...the organization of elements via atomic number showed properties (with a magical number of 8, of course) that were...wait for it...periodic! Elements in the same columns tend to behave the same way. We now know this is because of their valence electrons.
Because of periodicity, trends in elements can be predicted. We discuss four types of trends: ionization energy, electronegativity, atomic radius and ionic radius.
Mission Objective. You should be able to...
1. Explain the relationships between atomic number and ionization energy, atomic radius, electronegativity, and ionic radii.
...the organization of elements via atomic number showed properties (with a magical number of 8, of course) that were...wait for it...periodic! Elements in the same columns tend to behave the same way. We now know this is because of their valence electrons.
Because of periodicity, trends in elements can be predicted. We discuss four types of trends: ionization energy, electronegativity, atomic radius and ionic radius.
Atomic Radius (AR)
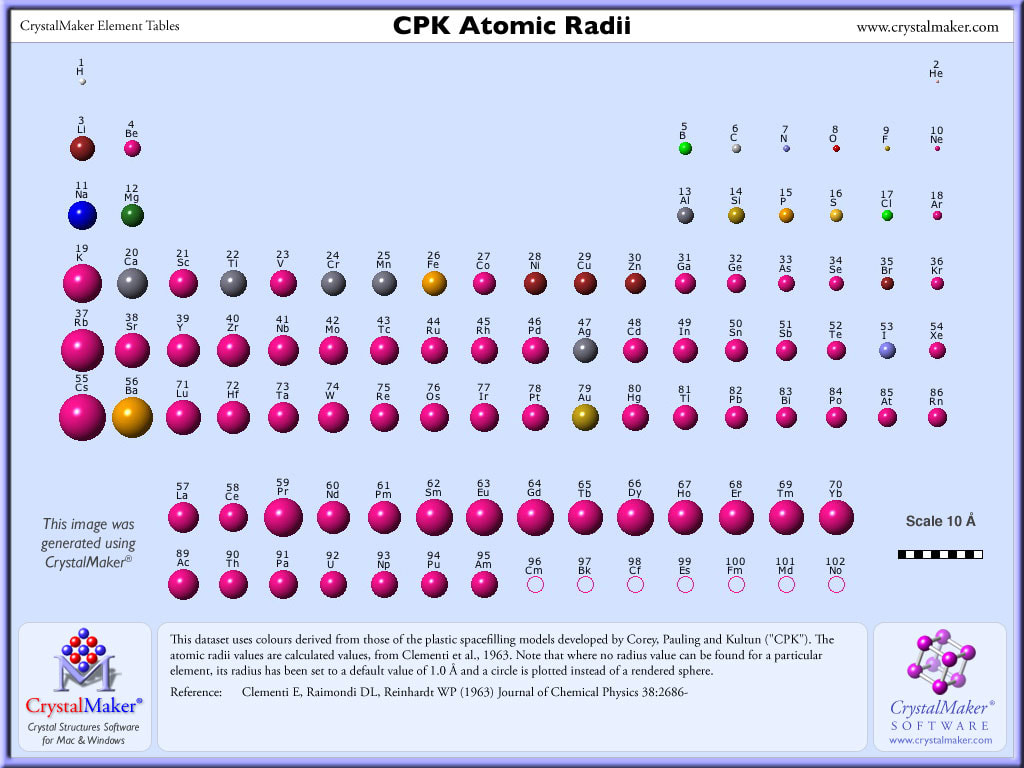
As atoms gain protons, their sizes decrease because of the increased positive pull of the nucleus on the valence electrons. Think in terms of a game of tug of war; with the nucleus on one end and the valence electrons on the other. Adding more protons pulls the valence electrons closer. Atomic number increases from left to right, so atoms decrease in size from left to right. Atomic number increases from top to bottom, so atoms increase in size from top to bottom.
Therefore, atomic radii decrease across a period and increase down a group.
As atoms gain protons, their sizes decrease because of the increased positive pull of the nucleus on the valence electrons. Think in terms of a game of tug of war; with the nucleus on one end and the valence electrons on the other. Adding more protons pulls the valence electrons closer. Atomic number increases from left to right, so atoms decrease in size from left to right. Atomic number increases from top to bottom, so atoms increase in size from top to bottom.
Therefore, atomic radii decrease across a period and increase down a group.
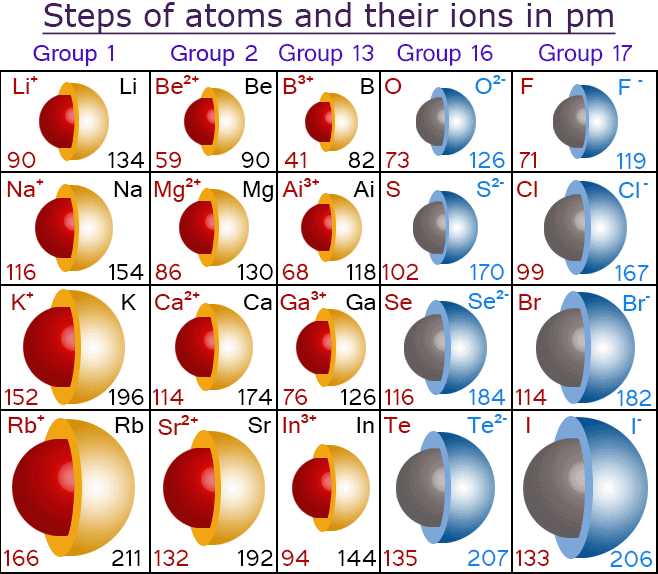
Ionic Radius (IR)
Metals form cations easily, and nonmetals form anions easily. Cations are always smaller than the neutral atoms from which they form, because the stripping of the valence electrons increases the attraction from the nucleus. As a result, the nucleus pulls the remaining electrons closer. Anions are always larger than the neutral atoms from which they form, because adding electrons decreases the nuclear pull.
Therefore, just like atomic radii, ionic radii decrease across a period and increase down a group.
Metals form cations easily, and nonmetals form anions easily. Cations are always smaller than the neutral atoms from which they form, because the stripping of the valence electrons increases the attraction from the nucleus. As a result, the nucleus pulls the remaining electrons closer. Anions are always larger than the neutral atoms from which they form, because adding electrons decreases the nuclear pull.
Therefore, just like atomic radii, ionic radii decrease across a period and increase down a group.
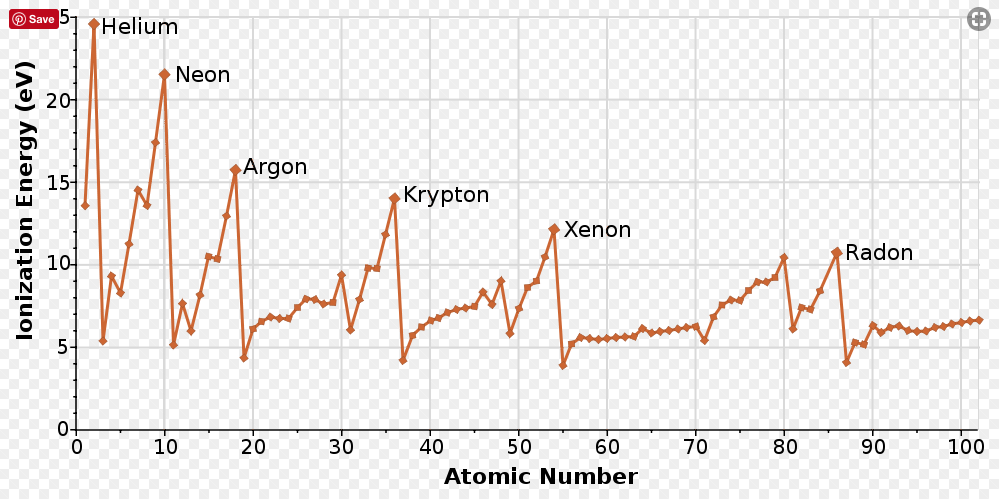
Ionization Energy (IE)
When an atom gains or loses an electron, it becomes an ion. Positively charged ions are called cations and negatively charged ions are called anions. The energy required to overcome the attraction of the nuclear charge and remove an electron is called ionization energy.
Removing one electron results in the formation of a cation with a +1 charge (and the energy required to do so is called the first ionization energy). Removing two electrons (which is more difficult) results in a cation with a +2 charge (second ionization energy). As the ionization energy increases, it becomes more and more difficult to remove electrons. Larger atoms' valence electrons are further away from the nucleus, and therefore have lower IEs.
Similarly, adding electrons increases the negative charge. Adding one electron results in the formation of an anion with a -1 charge. Adding two electrons results in an anion with a -2 charge, and so on and so forth.
Therefore, ionization energy increases across a period and decreases down a group.
When an atom gains or loses an electron, it becomes an ion. Positively charged ions are called cations and negatively charged ions are called anions. The energy required to overcome the attraction of the nuclear charge and remove an electron is called ionization energy.
Removing one electron results in the formation of a cation with a +1 charge (and the energy required to do so is called the first ionization energy). Removing two electrons (which is more difficult) results in a cation with a +2 charge (second ionization energy). As the ionization energy increases, it becomes more and more difficult to remove electrons. Larger atoms' valence electrons are further away from the nucleus, and therefore have lower IEs.
Similarly, adding electrons increases the negative charge. Adding one electron results in the formation of an anion with a -1 charge. Adding two electrons results in an anion with a -2 charge, and so on and so forth.
Therefore, ionization energy increases across a period and decreases down a group.
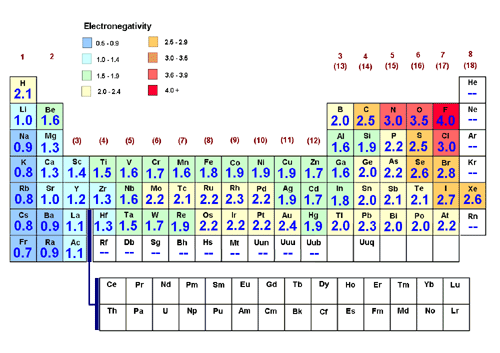
Electronegativity (EN)
The electronegativity of an element is the tendency of its atoms to attract electrons when they are chemically combined with another element. Noble gases do not have electronegativity values because they do not readily form compounds; they are inert. Electronegativity follows the same pattern as ionization energy and the opposite pattern of atomic radius.
Therefore, ionization energy increases across a period and decreases down a group.
The electronegativity of an element is the tendency of its atoms to attract electrons when they are chemically combined with another element. Noble gases do not have electronegativity values because they do not readily form compounds; they are inert. Electronegativity follows the same pattern as ionization energy and the opposite pattern of atomic radius.
Therefore, ionization energy increases across a period and decreases down a group.
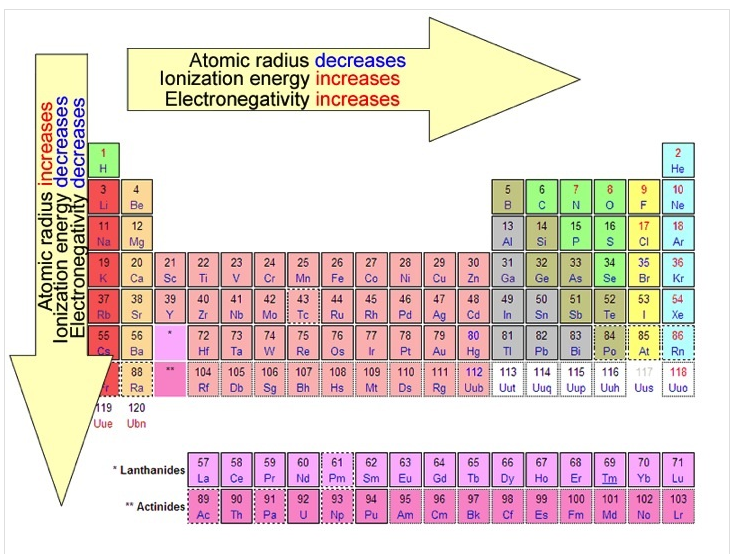
This summary image is courtesy of WilenskyChemistry.
Mission 3: Going Backwards.
Mission Objectives. You should be able to...
1. Briefly describe the arrangement of electrons in an atom.
As you're learning about atomic theory and the development of the atom, you should discover that most of the models do not specify the arrangement of electrons. JJ Thomson acknowledged their existence, but his model left a lot to be desired in terms of arrangement. Nagaoka's model is similar to Bohr's model in that the electrons orbit the positively charged nucleus. Bohr's model works for hydrogen, but for successively higher atomic numbers, the model fails. Bohr predicted that electrons orbit the nucleus in energy levels similar to how planets orbit the sun, a two-dimensional orbit.
However, we now know that electrons orbit the nucleus in 3-D regions called orbitals. The currently accepted atomic model, the quantum mechanical model, is a mathematical model that is based on the probability of locating an electron. 90% of the time, electrons can be found in these orbitals.
Below you have the s and p orbitals.
Mission Objectives. You should be able to...
1. Briefly describe the arrangement of electrons in an atom.
As you're learning about atomic theory and the development of the atom, you should discover that most of the models do not specify the arrangement of electrons. JJ Thomson acknowledged their existence, but his model left a lot to be desired in terms of arrangement. Nagaoka's model is similar to Bohr's model in that the electrons orbit the positively charged nucleus. Bohr's model works for hydrogen, but for successively higher atomic numbers, the model fails. Bohr predicted that electrons orbit the nucleus in energy levels similar to how planets orbit the sun, a two-dimensional orbit.
However, we now know that electrons orbit the nucleus in 3-D regions called orbitals. The currently accepted atomic model, the quantum mechanical model, is a mathematical model that is based on the probability of locating an electron. 90% of the time, electrons can be found in these orbitals.
Below you have the s and p orbitals.
Electrons obey three rules in their arrangement. You can read about them here. As atomic number increases, electrons fill energy levels and sublevels in an orderly fashion. The simplest sublevel is "S." The above image shows the S sublevel/orbital on the left. The image on the right is the P sublevel. You can look up D and F sublevels/orbitals. They're really funky-looking. As energy level increases, the size of the sublevel/orbital increases. Electrons in the outermost energy levels are called valence electrons. These electrons are the ones that determine chemical behavior.
Below are a series of videos that goes into detail about the behavior of electrons. We will watch each video and analyze the information. You're required to be familiar with the first 20 elements (H through Ca).
Below are a series of videos that goes into detail about the behavior of electrons. We will watch each video and analyze the information. You're required to be familiar with the first 20 elements (H through Ca).
Homework: Review the videos and take a look at this practice test. See if you can answer the questions. The test comes with an answer key so you can check your work. If there is a reason why you feel you can't answer a question, then that is a question you must ask me during class.
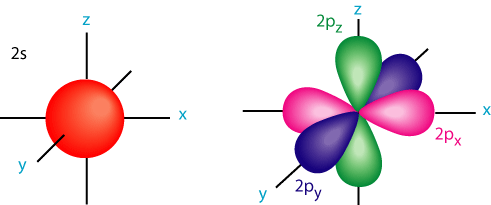
 RSS Feed
RSS Feed