You will do a lab next week experimenting with macromolecules. You will be testing for lipids, carbohydrates and proteins.
Go here and download this lab writeup. You should have it read by Wednesday, Aug. 23.
Different foods change color when indicators were added to denote the presence of macromolecules. Why does this happen? To understand this phenomenon, you need to be able to describe the structures of carbohydrates, lipids and proteins and explain why they behave in the way that they do when exposed to certain indicators.
You will have at least three (3) hypotheses. The hypotheses need to be tailored into "if...then" statements that are derived from the information presented in the Introduction. For example, "If nucleic acids are present in a sample, then adding phenolphthalein will turn the sample pink." You will complete three tests, so you should have three hypotheses.
Background Knowledge. Take a look at this interactive link. It describes the six functional groups. What is important for you to understand is what happens during the complementary processes of hydrolysis and dehydration. Another important fact is to understand what happens when electrons are donated by one molecule and received by another. Molecules that donate electrons are called reducing agents and molecules that receive electrons are reduced.
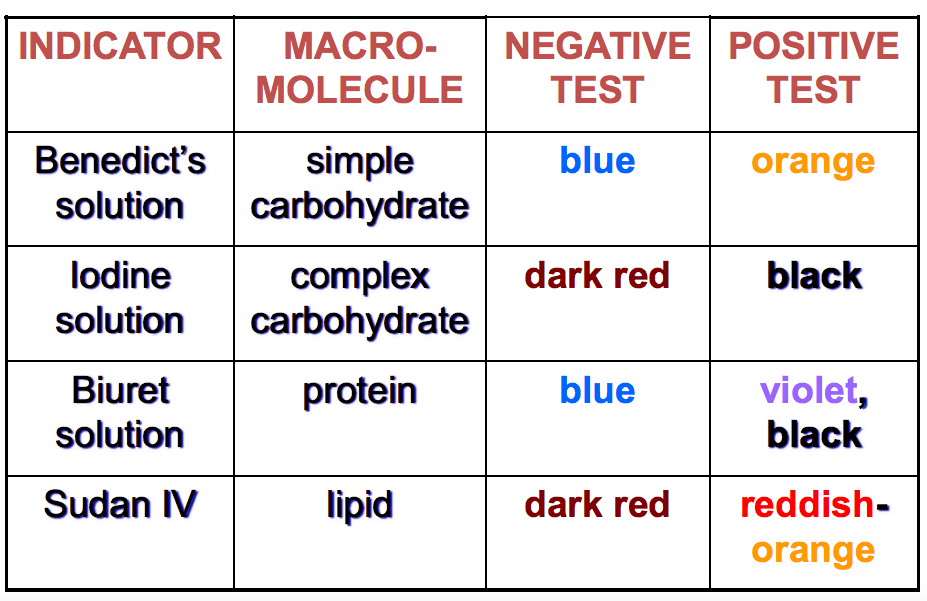
We will be working with three specific reagents: Benedict's reagent, Biuret's reagent, and iodine solution. To test for lipids, we will put the samples in water.
Benedict’s reagent changes color when exposed to a reducing agent, and all monosaccharides are reducing agents. Some disaccharides (like sucrose) have no free carbonyl groups and thus are non-reducing agents.
Biuret's reagent changes color in the presence of proteins because the copper II ions form a complex with the nitrogen atoms. The color change ranges from blue to violet, and the more peptide bonds present, the more violet the change.
Iodine solution is good for identifying the presence of starch because iodine atoms can fit inside the helical structure of starch compounds and change the color from dark blue to black.
If you want to know more (including the overwhelming biochemistry of it all, go here and here.
Go here and download this lab writeup. You should have it read by Wednesday, Aug. 23.
Different foods change color when indicators were added to denote the presence of macromolecules. Why does this happen? To understand this phenomenon, you need to be able to describe the structures of carbohydrates, lipids and proteins and explain why they behave in the way that they do when exposed to certain indicators.
You will have at least three (3) hypotheses. The hypotheses need to be tailored into "if...then" statements that are derived from the information presented in the Introduction. For example, "If nucleic acids are present in a sample, then adding phenolphthalein will turn the sample pink." You will complete three tests, so you should have three hypotheses.
Background Knowledge. Take a look at this interactive link. It describes the six functional groups. What is important for you to understand is what happens during the complementary processes of hydrolysis and dehydration. Another important fact is to understand what happens when electrons are donated by one molecule and received by another. Molecules that donate electrons are called reducing agents and molecules that receive electrons are reduced.
We will be working with three specific reagents: Benedict's reagent, Biuret's reagent, and iodine solution. To test for lipids, we will put the samples in water.
Benedict’s reagent changes color when exposed to a reducing agent, and all monosaccharides are reducing agents. Some disaccharides (like sucrose) have no free carbonyl groups and thus are non-reducing agents.
Biuret's reagent changes color in the presence of proteins because the copper II ions form a complex with the nitrogen atoms. The color change ranges from blue to violet, and the more peptide bonds present, the more violet the change.
Iodine solution is good for identifying the presence of starch because iodine atoms can fit inside the helical structure of starch compounds and change the color from dark blue to black.
If you want to know more (including the overwhelming biochemistry of it all, go here and here.
You need to create a chart for data collection. Remember that you will have three hypotheses. I expect you to run three trials. After you have collected your data, you need to determine if your hypotheses were supported by the data. I strongly suggest you show me your conclusions before you submit your work.
You will turn in your hypotheses, your data table, and your conclusion for a lab grade. Each person will submit their own report.
You will turn in your hypotheses, your data table, and your conclusion for a lab grade. Each person will submit their own report.
 RSS Feed
RSS Feed