Housekeeping: Well, we've made it through the first week!!!
Agenda:
1. Review
2. Macromolecules
3. Water
Lesson Objectives. By the end of this lesson, you will be able to:
1. Define and distinguish between the four classes of macromolecules
2. Compare and contrast the structure of macromolecules.
3. Name two kinds of each macromolecule (except nucleic acids).
4. Explain why carbon is an essential element in organic chemistry.
Content Review:
Links: Biochem Basics Macromolecules
Textbook Readings:
Student Missions:
Mission 1: Atoms & Molecules Review
Mission Objectives: You should be able to...
1. Recall the structure of the atom
2. Explain the difference between ionic bonding and covalent bonding.
Let's go here and watch a short video.
I'm giving you a handout to work on as we move through this unit.
Mission 2: Big Little Things.
Mission Objectives. You should be able to...
1. Define and distinguish between the four classes of macromolecules
2. Compare and contrast the structure of macromolecules.
3. Name three kinds of each macromolecule.
4. Explain why carbon is an essential element in organic chemistry.
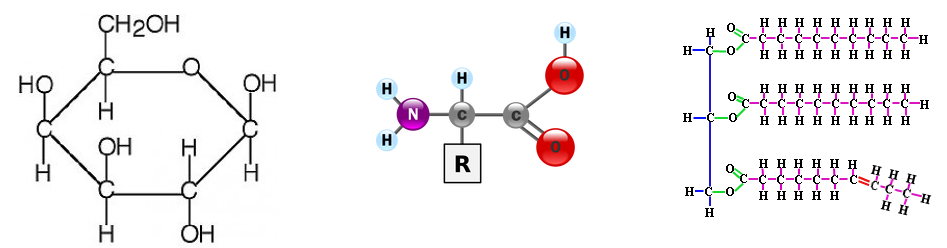
Carbon atoms can form four covalent bonds, which allows a diversity of stable compounds to exist. As a result, carbon-containing organic molecules can form chains, rings, and branched chains. This allows for the assembly of macromolecules.
There are four classes of macromolecules that make up living organisms: carbohydrates, lipids, proteins & nucleic acids. Each macromolecule has its own building blocks. Carbohydrates are composed of monosaccharides (simple sugars), proteins are composed of amino acids, lipids are composed of glycerol, fatty acids, and phosphate groups, and nucleic acids are formed from nucleotides.
The Amoeba Sisters break down the nitty gritty. You need to create a chart or some sort of table that compares and contrasts macromolecules. We will pause at certain points to make sure you understand what you need to know. One thing is to know the structures of each type. What is a functional group? Which macromolecules contain amino groups? Which macromolecules contain carboxyl groups? What are the properties of the functional groups?
Agenda:
1. Review
2. Macromolecules
3. Water
Lesson Objectives. By the end of this lesson, you will be able to:
1. Define and distinguish between the four classes of macromolecules
2. Compare and contrast the structure of macromolecules.
3. Name two kinds of each macromolecule (except nucleic acids).
4. Explain why carbon is an essential element in organic chemistry.
Content Review:
Links: Biochem Basics Macromolecules
Textbook Readings:
Student Missions:
Mission 1: Atoms & Molecules Review
Mission Objectives: You should be able to...
1. Recall the structure of the atom
2. Explain the difference between ionic bonding and covalent bonding.
Let's go here and watch a short video.
I'm giving you a handout to work on as we move through this unit.
Mission 2: Big Little Things.
Mission Objectives. You should be able to...
1. Define and distinguish between the four classes of macromolecules
2. Compare and contrast the structure of macromolecules.
3. Name three kinds of each macromolecule.
4. Explain why carbon is an essential element in organic chemistry.
Carbon atoms can form four covalent bonds, which allows a diversity of stable compounds to exist. As a result, carbon-containing organic molecules can form chains, rings, and branched chains. This allows for the assembly of macromolecules.
There are four classes of macromolecules that make up living organisms: carbohydrates, lipids, proteins & nucleic acids. Each macromolecule has its own building blocks. Carbohydrates are composed of monosaccharides (simple sugars), proteins are composed of amino acids, lipids are composed of glycerol, fatty acids, and phosphate groups, and nucleic acids are formed from nucleotides.
The Amoeba Sisters break down the nitty gritty. You need to create a chart or some sort of table that compares and contrasts macromolecules. We will pause at certain points to make sure you understand what you need to know. One thing is to know the structures of each type. What is a functional group? Which macromolecules contain amino groups? Which macromolecules contain carboxyl groups? What are the properties of the functional groups?
Mission 3: Well, It's What We're Made Of, Right?
Mission Objectives. You should be able to...
1. List and describe the essential properties of water.
2. Explain the necessity of hydrogen bonding.
3. Explain the solubility of water and its relationship to transporting molecules in organisms.
Water is the medium of life. Understanding why it is so important requires understanding of its structure. There are two hydrogen ions sharing electrons with an oxygen ion. Sharing of electrons results in a covalent bond. The water molecule is polar, meaning that it has a net charge on one end (the oxygen end). As a result, water can interact with itself and other molecules in different ways. Water has many different properties which makes it so important in living organisms.
Mission Objectives. You should be able to...
1. List and describe the essential properties of water.
2. Explain the necessity of hydrogen bonding.
3. Explain the solubility of water and its relationship to transporting molecules in organisms.
Water is the medium of life. Understanding why it is so important requires understanding of its structure. There are two hydrogen ions sharing electrons with an oxygen ion. Sharing of electrons results in a covalent bond. The water molecule is polar, meaning that it has a net charge on one end (the oxygen end). As a result, water can interact with itself and other molecules in different ways. Water has many different properties which makes it so important in living organisms.
For the next class, please come in with construction paper, glue, scissors, colored markers, etc. We will be making macromolecule models and personalized enzymes.

 RSS Feed
RSS Feed