Housekeeping: Thursday is a holiday, but it doesn't affect us.
Agenda:
Review Macromolecules
Carbohydrates & Lipids in detail
Review questions for Chunk #1.
Content Review:
Chapter 2 PowerPoint
Section 2.3
Macromolecules Functional Groups
Mad!Science!Links: 2.4, 2.5, & 2.6
Textbook Readings: Chapter 2, sections 2.4, 2.5, 2.6
Workbook Lessons: #48, #49, #50, #51, #53, #54
Student Missions:
Mission 1: Carbohydrates & Lipids
Mission Objectives. You should be able to...
1. Describe the structures of saccharides and provide examples.
2. Explain the differences between saturated and unsaturated fats.
3. Apply the structure of a triglyceride to its role in the human diet.
Let's go here.
Agenda:
Review Macromolecules
Carbohydrates & Lipids in detail
Review questions for Chunk #1.
Content Review:
Chapter 2 PowerPoint
Section 2.3
Macromolecules Functional Groups
Mad!Science!Links: 2.4, 2.5, & 2.6
Textbook Readings: Chapter 2, sections 2.4, 2.5, 2.6
Workbook Lessons: #48, #49, #50, #51, #53, #54
Student Missions:
Mission 1: Carbohydrates & Lipids
Mission Objectives. You should be able to...
1. Describe the structures of saccharides and provide examples.
2. Explain the differences between saturated and unsaturated fats.
3. Apply the structure of a triglyceride to its role in the human diet.
Let's go here.
G11 Bio Review questions; Sections 2.1, 2.2 & 2.3.
Outline condensation and hydrolysis reactions using a different example for each.
Outline the effect of temperature and substrate concentration on the activity of enzymes.
Outline the role of hydrolysis in the relationships between monosaccharides, disaccharides and polysaccharides.
Explain the importance of enzymes to human digestion.
State four elements that are needed by living organisms, other than carbon, hydrogen and oxygen, giving one role of each.
Compare the use of carbohydrates and lipids in energy storage.
Explain how the properties of water are significant to living organisms.
Mission 2: Proteins...The Meat Of It All.
Mission Objectives. You should be able to...
1. Define "polypeptide."
2. Explain how amino acids form proteins.
3. Describe how genes code for polypeptides.
4. List and describe the four protein structures.
5. Differentiate between proteome and genome.
Amino acids are monomers just like monosaccharides. They are building blocks for proteins just like monosaccharides are building blocks for carbohydrates. An amino acid contains an amino group and a carboxyl group attached to a central carbon atom with a terminal hydrogen and a variable group denoted as “R.” The R group is different for each amino acid, of which there are 20 naturally occurring. This allows for a wide variety of polypeptides. Each polypeptide that has a specific function has its own amino sequence and 3-D shape. Changing even one amino in the sequence can greatly alter the function of the polypeptide.
The 20 naturally occurring amino acids ("aminos") synthesize polypeptides under the control of DNA. Specifically, each polypeptide is created under the control of a specific gene. Think back to differentiation, which is a less specialized cell type becoming more specialized. This is done under the coding of specific genes. Some genes are universal, such as the ones that code for common proteins such as ribosomes.
Outline condensation and hydrolysis reactions using a different example for each.
Outline the effect of temperature and substrate concentration on the activity of enzymes.
Outline the role of hydrolysis in the relationships between monosaccharides, disaccharides and polysaccharides.
Explain the importance of enzymes to human digestion.
State four elements that are needed by living organisms, other than carbon, hydrogen and oxygen, giving one role of each.
Compare the use of carbohydrates and lipids in energy storage.
Explain how the properties of water are significant to living organisms.
Mission 2: Proteins...The Meat Of It All.
Mission Objectives. You should be able to...
1. Define "polypeptide."
2. Explain how amino acids form proteins.
3. Describe how genes code for polypeptides.
4. List and describe the four protein structures.
5. Differentiate between proteome and genome.
Amino acids are monomers just like monosaccharides. They are building blocks for proteins just like monosaccharides are building blocks for carbohydrates. An amino acid contains an amino group and a carboxyl group attached to a central carbon atom with a terminal hydrogen and a variable group denoted as “R.” The R group is different for each amino acid, of which there are 20 naturally occurring. This allows for a wide variety of polypeptides. Each polypeptide that has a specific function has its own amino sequence and 3-D shape. Changing even one amino in the sequence can greatly alter the function of the polypeptide.
The 20 naturally occurring amino acids ("aminos") synthesize polypeptides under the control of DNA. Specifically, each polypeptide is created under the control of a specific gene. Think back to differentiation, which is a less specialized cell type becoming more specialized. This is done under the coding of specific genes. Some genes are universal, such as the ones that code for common proteins such as ribosomes.
Ribosomes connect amino acids to make proteins. Condensation reactions allow the process to take place. The process produces a peptide bond that creates a protein and a water molecule. Peptide bonds combines two amino acids to make a dipeptide. A chain of amino acids forms a polypeptide, and most proteins are polypeptides.
Go to the summary page and take a look at the four different types of protein structures. Be able to describe them in words.
Genomes & Proteomes. The specific DNA sequence that is unique to an individual is called a genome. This means that each individual has a unique set of proteins that s/he is capable of synthesizing. Thus, each individual is said to have a unique proteome as well as a unique genome. Proteomes are all the proteins produced by a cell, tissue or organism. The genome is all of the genes of a cell, tissue or organism. The genome is fixed, but the proteome is variable.
Denaturization. The intramolecular bonds that hold together secondary, tertiary, and quaternary structures are susceptible to alterations in temperature and pH. The bonds that hold the structure together are susceptible to alterations in temperature and pH. When the proteins are placed into an environment that is higher than optimal levels, the increased vibration of the molecules breaks the fragile hydrogen bonds that hold the structure in place. When the pH changes (with either too many H+ ions or OH- ions), the extra charges prevents normal H bonding, so the shape cannot form.
Homework:
Using either the summary webpage or the text as a guide, create models of each protein structure using colored paper (or paper that has been colored). Each structure should be an actual protein and you should be able to name it and describe its function. You should also be able to answer the question: Why do you think the structure is as simple/complex as it is and how does that relate to the protein's function?
Here is the rubric.
Go to the summary page and take a look at the four different types of protein structures. Be able to describe them in words.
Genomes & Proteomes. The specific DNA sequence that is unique to an individual is called a genome. This means that each individual has a unique set of proteins that s/he is capable of synthesizing. Thus, each individual is said to have a unique proteome as well as a unique genome. Proteomes are all the proteins produced by a cell, tissue or organism. The genome is all of the genes of a cell, tissue or organism. The genome is fixed, but the proteome is variable.
Denaturization. The intramolecular bonds that hold together secondary, tertiary, and quaternary structures are susceptible to alterations in temperature and pH. The bonds that hold the structure together are susceptible to alterations in temperature and pH. When the proteins are placed into an environment that is higher than optimal levels, the increased vibration of the molecules breaks the fragile hydrogen bonds that hold the structure in place. When the pH changes (with either too many H+ ions or OH- ions), the extra charges prevents normal H bonding, so the shape cannot form.
Homework:
Using either the summary webpage or the text as a guide, create models of each protein structure using colored paper (or paper that has been colored). Each structure should be an actual protein and you should be able to name it and describe its function. You should also be able to answer the question: Why do you think the structure is as simple/complex as it is and how does that relate to the protein's function?
Here is the rubric.
Mission 3: Enzymes! Catalyzing Reactions Since...Forever!!!
Misson Objectives. You should be able to...
1. Sketch and describe how enzymes and substrates bind together.
2. Graphically represent the effects of temperature, pH and concentration on enzyme activity.
3. Define "denaturation" and describe the process as it relates to enzymes.
4. Explain how enzymes are used in industry.
Make sure you pay attention to the effects of temperature, pH and concentration on enzyme activity. Try to sketch a graph of what each effect would look like.
Take a look at this animation.
Misson Objectives. You should be able to...
1. Sketch and describe how enzymes and substrates bind together.
2. Graphically represent the effects of temperature, pH and concentration on enzyme activity.
3. Define "denaturation" and describe the process as it relates to enzymes.
4. Explain how enzymes are used in industry.
Make sure you pay attention to the effects of temperature, pH and concentration on enzyme activity. Try to sketch a graph of what each effect would look like.
Take a look at this animation.
HOMEWORK:
Get to know enzymes on an interactive level with Bioman's Enzymatic Game. Complete the interactive and take the quiz. You only get one chance. Screen-shot your quiz score and send it to me at [email protected].
Mission 4: The Building Blocks of Building Blocks.
Mission Objectives. You should be able to...
1. Describe the structure of DNA and RNA.
2. Compare and contrast the structures of DNA and RNA.
3. Sketch and annotate both structures and explain what 5' and 3' mean.
4. Define and distinguish betweens purines and pyrimidines.
Just like monosaccharides are the building blocks for carbohydrates and amino acids are the building blocks for proteins, nucleotides are building blocks for nucleic acids. There are three primary examples of nucleic acids: ATP, DNA and RNA. They are polymers (long chains) of nucleotides.
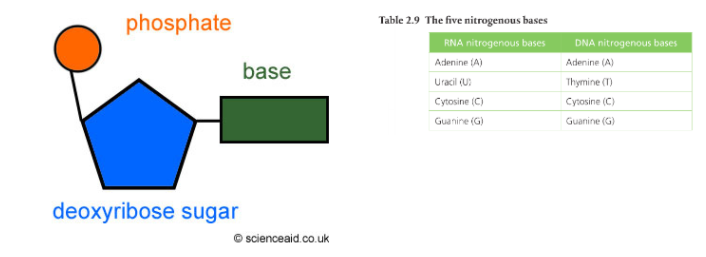
A nucleotide has three parts: a phosphate group, a nitrogenous base, and a 5-carbon sugar at its center. The bonds holding a nucleotide together are covalent.
The following diagram is a standard general representation of a nucleotide. Phosphate groups are circles, the sugars are pentagons, and the nitrogenous bases are rectangles.
Get to know enzymes on an interactive level with Bioman's Enzymatic Game. Complete the interactive and take the quiz. You only get one chance. Screen-shot your quiz score and send it to me at [email protected].
Mission 4: The Building Blocks of Building Blocks.
Mission Objectives. You should be able to...
1. Describe the structure of DNA and RNA.
2. Compare and contrast the structures of DNA and RNA.
3. Sketch and annotate both structures and explain what 5' and 3' mean.
4. Define and distinguish betweens purines and pyrimidines.
Just like monosaccharides are the building blocks for carbohydrates and amino acids are the building blocks for proteins, nucleotides are building blocks for nucleic acids. There are three primary examples of nucleic acids: ATP, DNA and RNA. They are polymers (long chains) of nucleotides.
A nucleotide has three parts: a phosphate group, a nitrogenous base, and a 5-carbon sugar at its center. The bonds holding a nucleotide together are covalent.
The following diagram is a standard general representation of a nucleotide. Phosphate groups are circles, the sugars are pentagons, and the nitrogenous bases are rectangles.
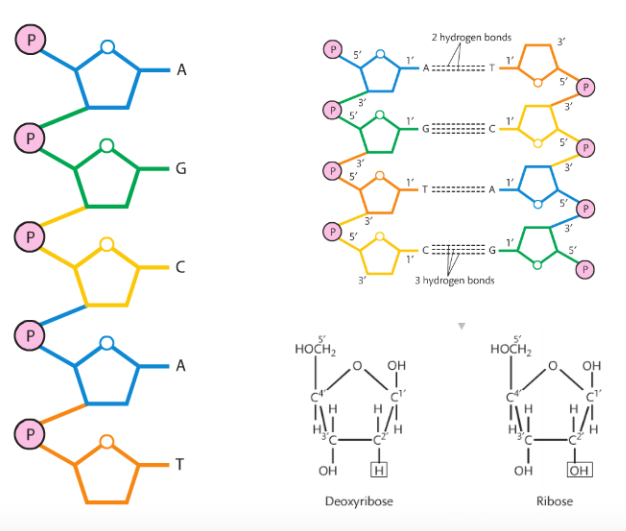
The phosphate groups are the same in both DNA and RNA, but the sugars are different (DNA has deoxyribose and RNA has ribose). The nitrogenous bases are slightly different: uracil is found only in RNA and thymine is only in DNA. The other bases (adenine, guanine, and cytosine) are found in both nucleic acids. Condensation reactions allow the nucleotides to bond to one another and form a chain.
RNA is single stranded and DNA is double stranded.
RNA is single stranded and DNA is double stranded.
The 5-carbon sugars have the same orientation in RNA, but in order for DNA to be a double helix, its strands must be anti-parallel. Look at the molecule and you'll see the left strand has deoxyribose with the carbon atom at the top of the pentagon. This is called the 5' (five prime). On the right strand, the pentagon is upside down and now it is referred to as 3' (three prime).
Homework: Below.
Homework: Below.
 RSS Feed
RSS Feed