Mission 1: Charge it Up!
Mission Objectives. You should be able to...
1. Predict whether sigma or pi bonds are formed from the linear combination of atomic orbitals.
2. Deduce the Lewis structures of molecules and ions showing all valence electrons for up to six electron pairs on each atom.
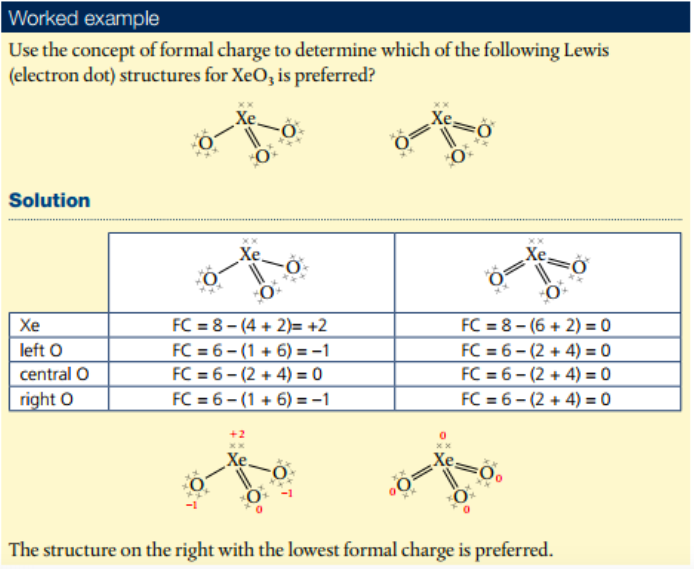
3. Apply Formal Charge to ascertain which Lewis structure is preferred from different Lewis structures.
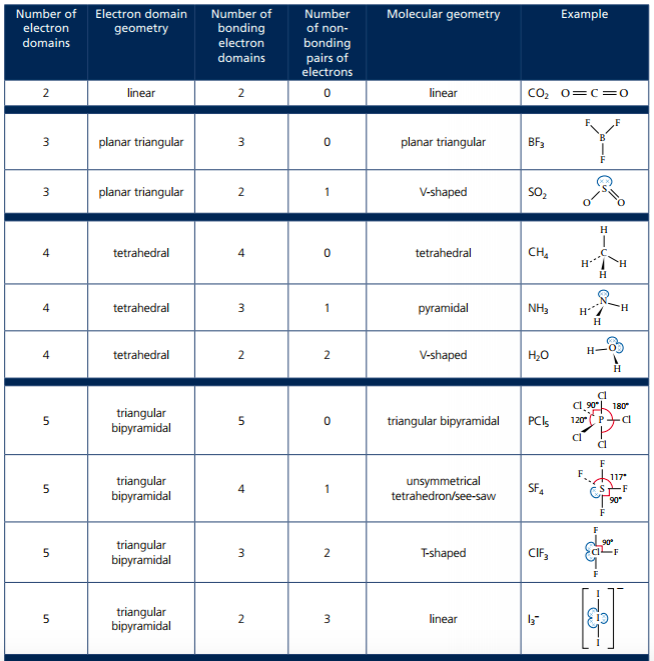
4. Deduce using VSEPR Theory of the electron domain geometry and molecular geometry with five and six electron domains and associated bond angles.
Mission Objectives. You should be able to...
1. Predict whether sigma or pi bonds are formed from the linear combination of atomic orbitals.
2. Deduce the Lewis structures of molecules and ions showing all valence electrons for up to six electron pairs on each atom.
3. Apply Formal Charge to ascertain which Lewis structure is preferred from different Lewis structures.
4. Deduce using VSEPR Theory of the electron domain geometry and molecular geometry with five and six electron domains and associated bond angles.
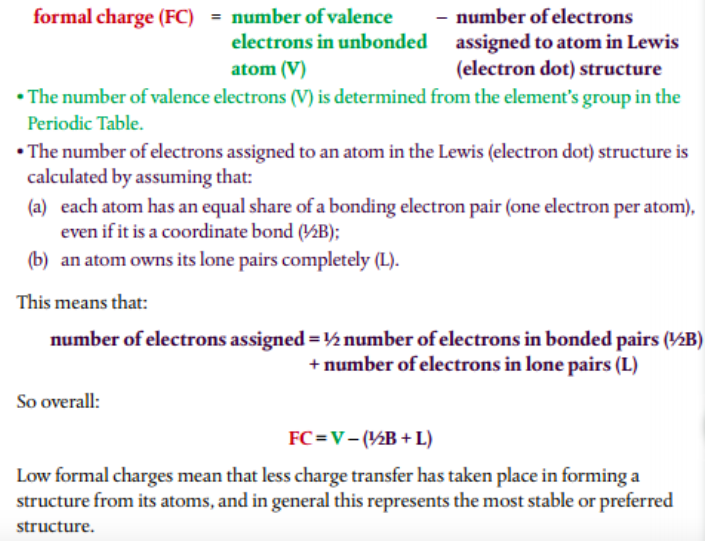
Formal charge is a way to keep up with the available electrons in a structural formula. The way to calculate formal charge is as follows:
# of valence electrons - 1/2 # of bonding electrons - # of free electrons.
# of valence electrons - 1/2 # of bonding electrons - # of free electrons.
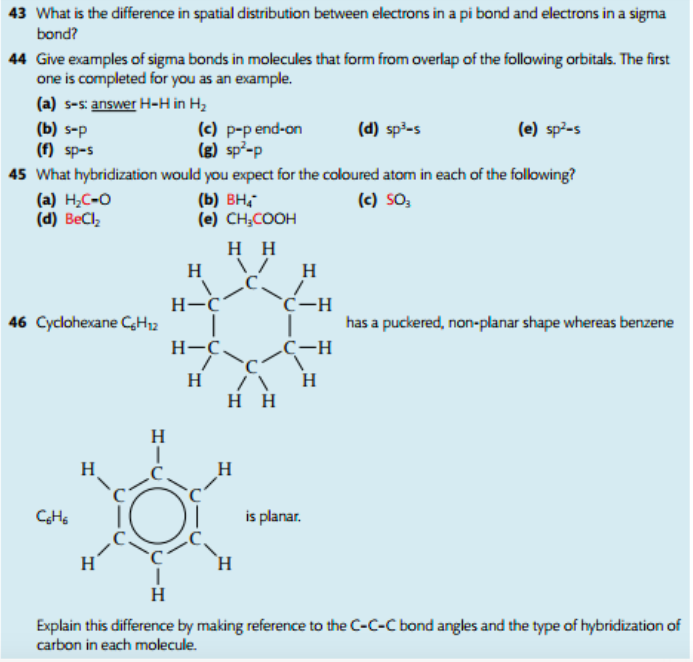
Expanded Octets. When the central atom is an element from the 3rd period or below, compounds can form with more than eight electrons around the central atom. This is called an expanded octet. This arrangement is possible because the d orbitals available in the valence shell of these atoms have energy values relatively close to those of the p orbitals. Promoting electrons from 3p to empty 3d orbitals allows additional electron pairs to form. This happens with phosphorous and sulfur.
For example: Phosphorous pentachloride has five electron domains and forms a shape called trigonal bipyramidal. It contains three different bond angles: 90, 120, and 180 degrees.
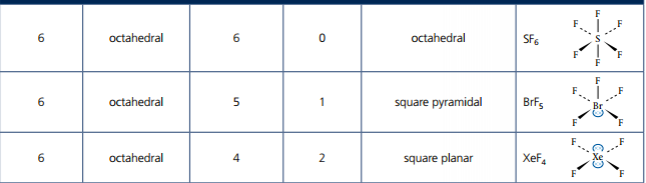
There are tables on pages 333 and 334 that illustrate the shape and bond angles for molecules with expanded octets. Lone pairs of electrons occupy equatorial positions. Basically, just count the number of electron pairs on the central atom, both shared and lone. If there are five pairs of electrons total, then that is 5 electron domains that form a trigonal bipyramidal shape. Six pairs of electrons around the central atom leads to an octahedral shape. There are other shapes to get familiar with: see-saw, linear, T-shape, square planar and square pyramidal.
For example: Phosphorous pentachloride has five electron domains and forms a shape called trigonal bipyramidal. It contains three different bond angles: 90, 120, and 180 degrees.
There are tables on pages 333 and 334 that illustrate the shape and bond angles for molecules with expanded octets. Lone pairs of electrons occupy equatorial positions. Basically, just count the number of electron pairs on the central atom, both shared and lone. If there are five pairs of electrons total, then that is 5 electron domains that form a trigonal bipyramidal shape. Six pairs of electrons around the central atom leads to an octahedral shape. There are other shapes to get familiar with: see-saw, linear, T-shape, square planar and square pyramidal.
Below is a short video that talks about delocalization. Basically, if a molecule has resonance, delocalization occurs and stabilizes the molecule.
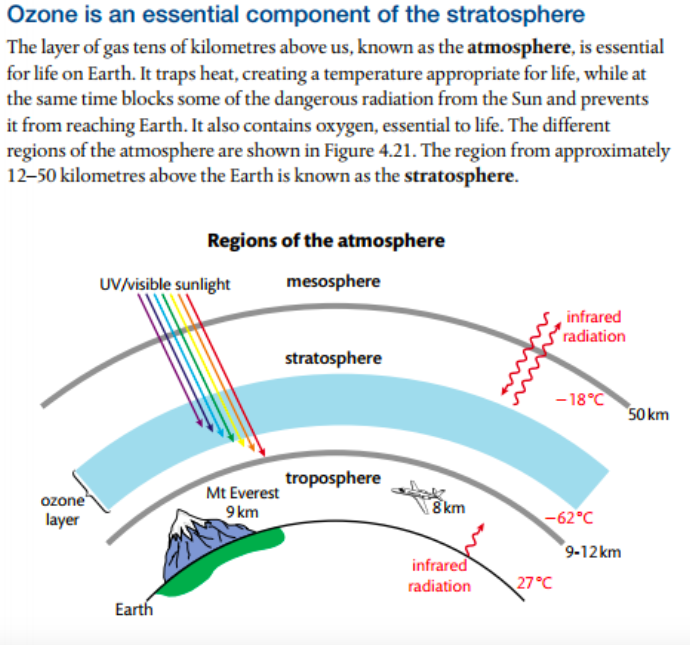
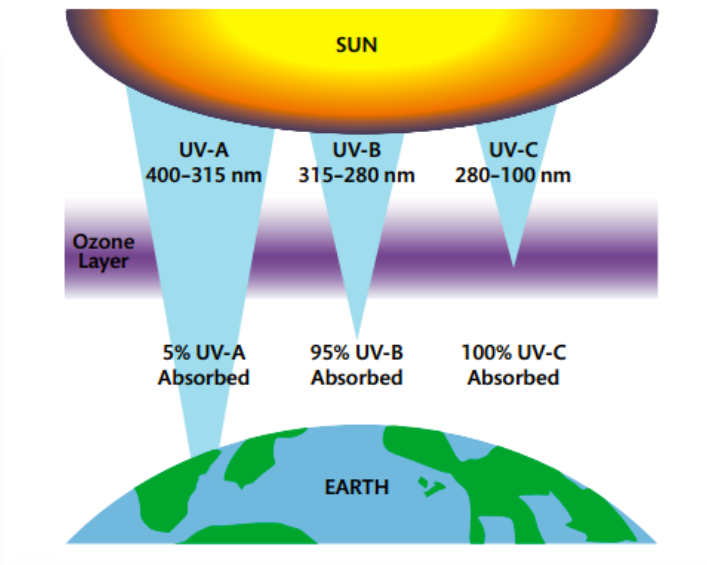
Mission 2: Ozone Depletion
Mission Objectives. You should be able to...
1. Explain the wavelength of light required to dissociate oxygen and ozone.
2. Describe the mechanism of the catalysis of ozone depletion when catalyzed by CFCs and NOx.
Mission Objectives. You should be able to...
1. Explain the wavelength of light required to dissociate oxygen and ozone.
2. Describe the mechanism of the catalysis of ozone depletion when catalyzed by CFCs and NOx.
Mission 3: Hybridization
Mission Objectives. You should be able to...
1. Explain how sp3, sp2 and sp hybrid orbitals are formed in methane, ethene and ethyne.
2. Identify and explain the relationship between Lewis structure, electron domains, molecular geometry, and types of hybridization.
Hybridization. A hybrid orbital results from the mixing of different types of atomic orbitals on the same atom. There are three types of hybridization: sp3 (ex: methane), sp2 (ex: ethene), and sp (ex. ethyne). For these examples, the second electron in carbon's 2s orbital gets excited to fill in the 2pz orbital. As a result, the 2s and 2p combine to form a set of orbitals.
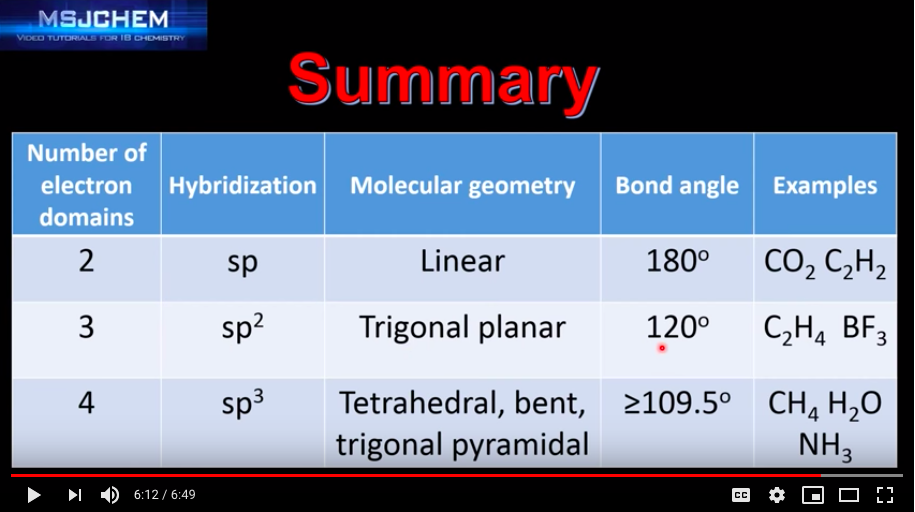
Molecules that have tetrahedral geometry are predicted to have sp3 hybridization. This means that there is a combination of 1 s orbital and 3 p orbitals. The molecule has 25% s character and 75% p character.
Molecules that have trigonal planar geometry are predicted to have sp2 hybridization. This means that there is a combination of 1 s orbital and 2 p orbitals. The molecule has 33% s character and 66.7% p character.
Molecules that have linear geometry are predicted to have sp hybridization. This is a combination of 1 s and 1 p orbital. The molecule has 50% s character and 50% p character. See the table on page 352.
Mission Objectives. You should be able to...
1. Explain how sp3, sp2 and sp hybrid orbitals are formed in methane, ethene and ethyne.
2. Identify and explain the relationship between Lewis structure, electron domains, molecular geometry, and types of hybridization.
Hybridization. A hybrid orbital results from the mixing of different types of atomic orbitals on the same atom. There are three types of hybridization: sp3 (ex: methane), sp2 (ex: ethene), and sp (ex. ethyne). For these examples, the second electron in carbon's 2s orbital gets excited to fill in the 2pz orbital. As a result, the 2s and 2p combine to form a set of orbitals.
Molecules that have tetrahedral geometry are predicted to have sp3 hybridization. This means that there is a combination of 1 s orbital and 3 p orbitals. The molecule has 25% s character and 75% p character.
Molecules that have trigonal planar geometry are predicted to have sp2 hybridization. This means that there is a combination of 1 s orbital and 2 p orbitals. The molecule has 33% s character and 66.7% p character.
Molecules that have linear geometry are predicted to have sp hybridization. This is a combination of 1 s and 1 p orbital. The molecule has 50% s character and 50% p character. See the table on page 352.


 RSS Feed
RSS Feed