Mission 1: Rate Expression & Reaction Mechanisms
Mission Objectives. You should be able to...
1. Deduce the rate equation from experimental data and solving problems involving the rate equation.
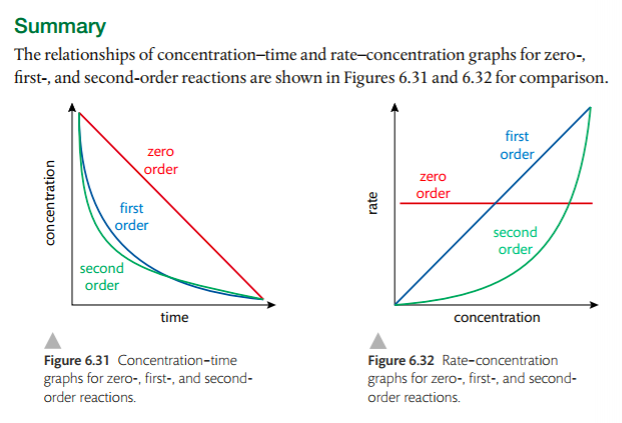
2. Sketch, identify and analyze graphical representations for zero, first and second order reactions.
3. Evaluate proposed reaction mechanisms to be consistent with kinetic & stoichiometric data.
Looking at the image below, a, b, c and d are stoichiometric coefficients. A, B, C & D are substance concentrations. The rate of a reaction depends on the concentrations of the reactants.
Image courtesy of mikeblaber.org.
Mission Objectives. You should be able to...
1. Deduce the rate equation from experimental data and solving problems involving the rate equation.
2. Sketch, identify and analyze graphical representations for zero, first and second order reactions.
3. Evaluate proposed reaction mechanisms to be consistent with kinetic & stoichiometric data.
Looking at the image below, a, b, c and d are stoichiometric coefficients. A, B, C & D are substance concentrations. The rate of a reaction depends on the concentrations of the reactants.
Image courtesy of mikeblaber.org.
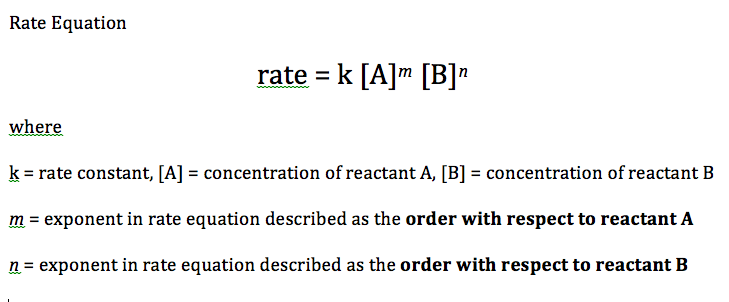
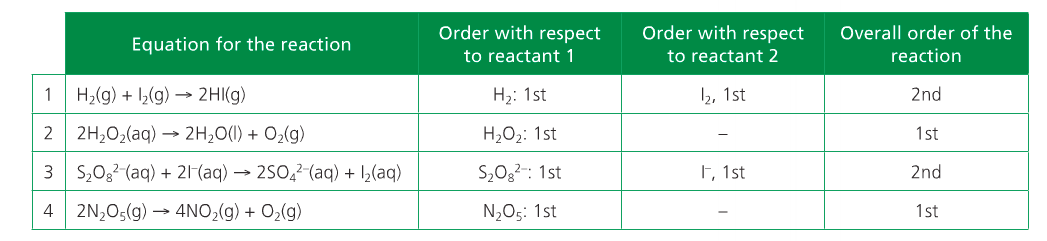
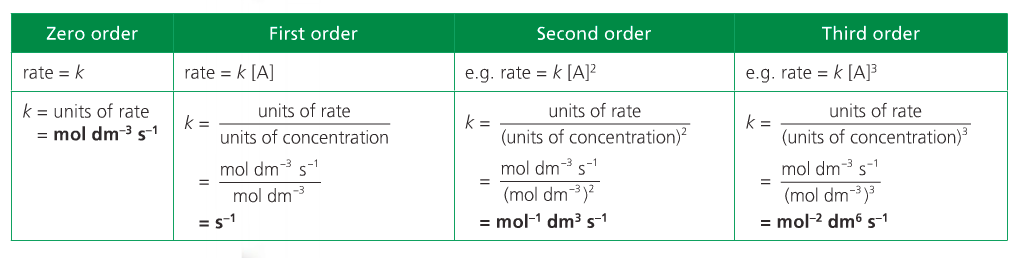
The overall order of the reaction is defined as the sum of the m and n exponents. Rate equations can only be determined experimentally because orders can only be deduced empirically.
The word "order" conveys how sensitive the rate of reaction is to changes in the concentrations of A and B.
The word "order" conveys how sensitive the rate of reaction is to changes in the concentrations of A and B.
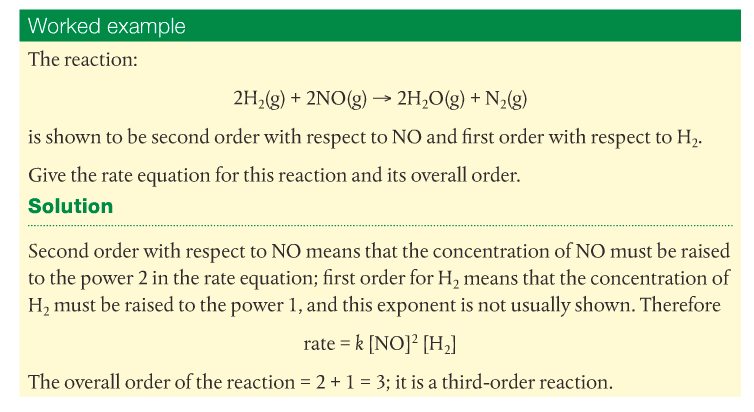
Here is a worked example from the Pearson text. Attempt to do the problems in the blue box.
I know there are a lot of videos, but they are all useful and each video explains the content in slightly different ways. It's worth it to watch them all (I do).
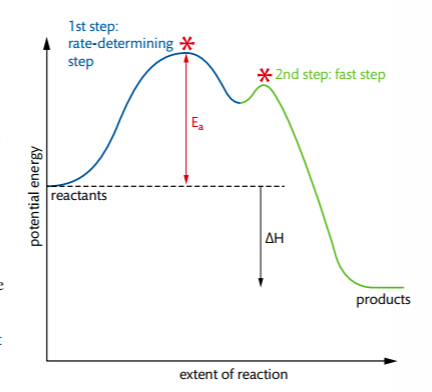
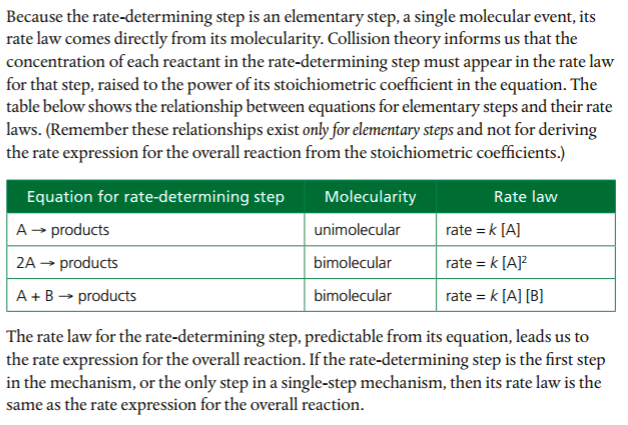
The sequence of reaction steps outlining the reaction pathway from reactants to the formation of products is called the reaction mechanism. Individual steps in reaction mechanisms are called elementary step, reaction or process. Elementary steps are classified by its molecularity, which represents the number of molecules or atoms involved as reactants in the elementary step.
Unimolecular: single molecule involved.
Bimolecular: two molecules or atoms involved in collision
Termolecular: three molecules or atoms involved in collision.
Each elementary step has its own rate constant, k, and its own activation energy.
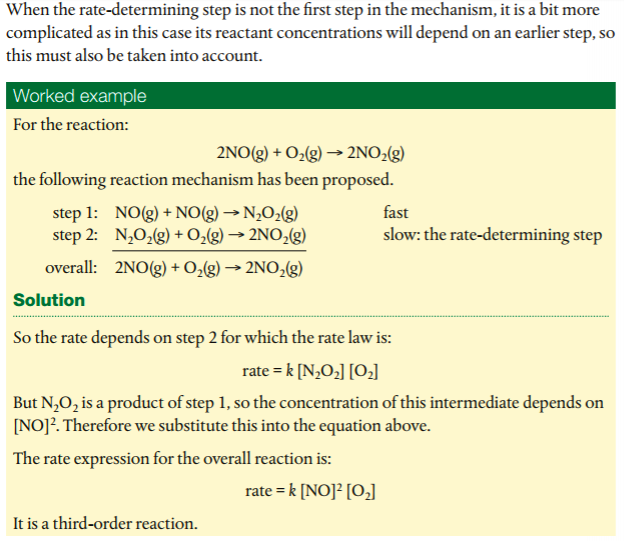
In order to deduce the rate equation from a proposed reaction mechanism, (1) decide which step is the rate-determining step (the rate of the overall reaction is equal to the rate of the slow step), and (2), from #1, deduce the rate equation for the rate determining step.
The awesomely named Mike Sugiyama Jones breaks it down.
Unimolecular: single molecule involved.
Bimolecular: two molecules or atoms involved in collision
Termolecular: three molecules or atoms involved in collision.
Each elementary step has its own rate constant, k, and its own activation energy.
In order to deduce the rate equation from a proposed reaction mechanism, (1) decide which step is the rate-determining step (the rate of the overall reaction is equal to the rate of the slow step), and (2), from #1, deduce the rate equation for the rate determining step.
The awesomely named Mike Sugiyama Jones breaks it down.
In this video, the instructor shows you how to suggest a mechanism using the rate law and how to determine the RDS.
Mission 2: Activation Energy
Mission Objectives. You should be able to...
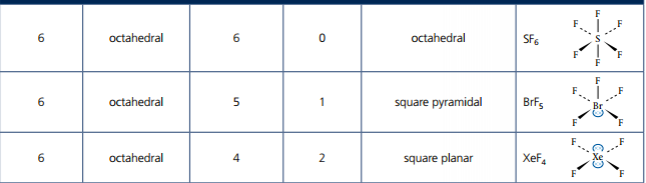
1. Analyze graphical representations of the Arrhenius equation in its linear form.
2. Use the Arrhenius equation.
3. Describe the relationships between temperature and rate constant, frequency factor, and complexity of molecules colliding.
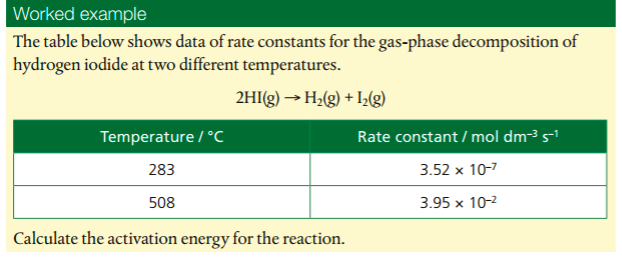
4. Determine and evaluate values of activation energy and frequency factors from data.
Image courtesy of IB Alchemy.
Mission Objectives. You should be able to...
1. Analyze graphical representations of the Arrhenius equation in its linear form.
2. Use the Arrhenius equation.
3. Describe the relationships between temperature and rate constant, frequency factor, and complexity of molecules colliding.
4. Determine and evaluate values of activation energy and frequency factors from data.
Image courtesy of IB Alchemy.
What is the effect of increased temperature on rate constant k? If you examine a Maxwell-Boltzmann distribution, particles at a higher temperature tend to have more collisions because the energy is greater than the activation energy. For most reactions, an increase in temperature of 10K doubles the rate of reaction. In the Arrhenius equation, e to the power of (-Ea/RT) is the fraction of molecules which have an energy equal to or greater than Ea at a particular temperature. So an increase in temperature increases the value of the rate constant k, and therefore the rate of reaction.
Watch the video. At the end, he does some practice problems. Put the calculations in your notes so you have a reference.
Watch the video. At the end, he does some practice problems. Put the calculations in your notes so you have a reference.
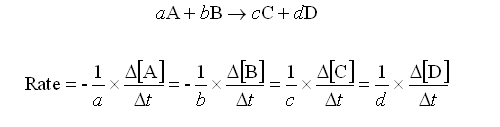
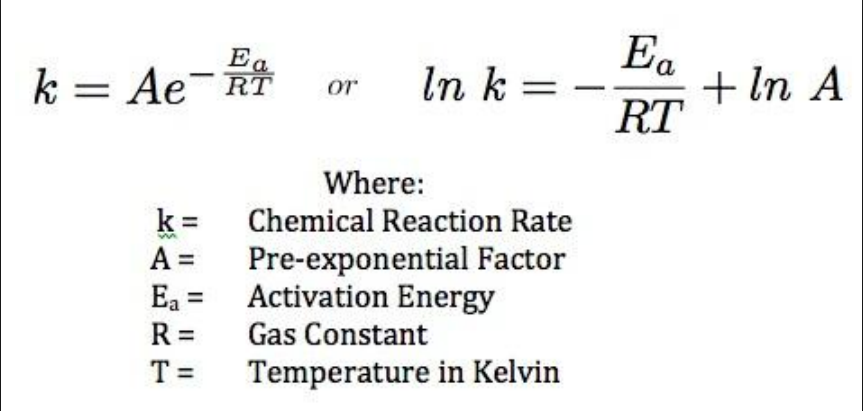




 RSS Feed
RSS Feed