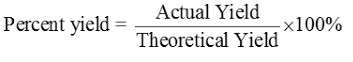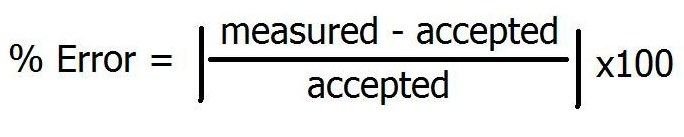Stoichiometry
Housekeeping: Welcome back! I hope you guys had a great holiday. We are going to spend the next few weeks on stoichiometry. Stoichiometry is a method that uses relationships between reactants and products in a chemical reaction to determine desired quantitative data.
Before we get into stoichiometry, we need to introduce the concept of the mole.
Before we get into stoichiometry, we need to introduce the concept of the mole.
Mission 1: The Mole
Mission Objectives. By the end of this lesson, you should be able to:
1. Define the "mole."
2. Calculate the molar mass of a compound.
3. Solve simple mole conversion problems.
Content Review:
Text: Chapter 1
1.1 The Particulate Nature of Matter
Links: Chemical Equations Stoichiometric Relationships KOGNITY
NOTICE: YOU WILL NEED YOUR CALCULATORS AND PERIODIC TABLES FOR THIS UNIT.
The mole is the SI unit for counting particles. One mole of any substance contains 6.02 * 10^23 particles. This number is known as Avogadro's Number, named after Italian scientist Amedeo Avogadro.
"Particles" is a generic term for atoms, formula units, and molecules. Avogadro's number of atoms clearly references an element, Avogadro's number of formula units references an ionic compound, and Avogadro's number of molecules references a covalent compound.
Mission Objectives. By the end of this lesson, you should be able to:
1. Define the "mole."
2. Calculate the molar mass of a compound.
3. Solve simple mole conversion problems.
Content Review:
Text: Chapter 1
1.1 The Particulate Nature of Matter
Links: Chemical Equations Stoichiometric Relationships KOGNITY
NOTICE: YOU WILL NEED YOUR CALCULATORS AND PERIODIC TABLES FOR THIS UNIT.
The mole is the SI unit for counting particles. One mole of any substance contains 6.02 * 10^23 particles. This number is known as Avogadro's Number, named after Italian scientist Amedeo Avogadro.
"Particles" is a generic term for atoms, formula units, and molecules. Avogadro's number of atoms clearly references an element, Avogadro's number of formula units references an ionic compound, and Avogadro's number of molecules references a covalent compound.
The molar mass of a substance is either the element's atomic mass on the periodic table, or the sum of the atomic masses of a formula unit or a compound.
For example: Hydrogen's molar mass is 1.01 g/mol. But water, which is H2O, is 18.02 g/mol. The 18.02 comes from adding the two hydrogens (2.02) with oxygen (rounded up to 16.00). So whenever you are dealing with the molar mass of a compound, you must add up the atomic mass of all of the atoms in that compound.
We will practice. Get comfortable with this process, as it is significant for solving stoichiometric problems.
Download these practice problems.
1. Molar Mass 2. Mole Conversions (I) 3. Mole Conversions (II)
Let's Practice!
Below is an overview of Chapter 1. I suggest you watch this a couple of times, as there is a lot crammed into one chapter.
For example: Hydrogen's molar mass is 1.01 g/mol. But water, which is H2O, is 18.02 g/mol. The 18.02 comes from adding the two hydrogens (2.02) with oxygen (rounded up to 16.00). So whenever you are dealing with the molar mass of a compound, you must add up the atomic mass of all of the atoms in that compound.
We will practice. Get comfortable with this process, as it is significant for solving stoichiometric problems.
Download these practice problems.
1. Molar Mass 2. Mole Conversions (I) 3. Mole Conversions (II)
Let's Practice!
Below is an overview of Chapter 1. I suggest you watch this a couple of times, as there is a lot crammed into one chapter.
Mission 2: Real Chemistry
Mission Objectives. You should be able to...
1. Observe and describe the reactants and products of a chemical reaction.
2. Determine the percent yield of the reaction.
3. Explain whether or not mass was conserved.
As we've been talking about, chemical reactions summarize chemical change and are represented by chemical equations. Reactants are on the left and products are on the right. Equations are balanced using coefficients to ensure that mass is conserved (we can't break that law, y'all). State symbols are used to denote the state of matter each reactant and product are in at the time of reaction.
Reminder: (s) = solid (l) = liquid (g) = gaseous (aq) = aqueous (dissolved in water)
Having said all that, let's take a look at some chemical reactions. You need to predict the results, measure the amount of reactants that you used and determine how much product you should have obtained. Here is the writeup.
The balanced chemical equation represents what is theoretically possible when a reaction is carried out under ideal conditions. It allows for the expected amount of products to be calculated. This is called the theoretical yield (TY). The objective is to get as close to the TY as possible. But we don't live in a perfect world and many factors, especially in large-scale processes, result in a reduced yield of products. Page 23 in your text lists some factors. This reduced yield is called either the experimental yield or actual yield (AY). You need these two values to determine the percentage yield of the actual process. The formula is below.
Percent error determines the precision of your experiment. The formula is below. You want your percent error to be as close to 0.00% as possible, as that means you are close to the accepted value for the experiment.
You will be expected to perform these calculations with every experiment.
Mission Objectives. You should be able to...
1. Observe and describe the reactants and products of a chemical reaction.
2. Determine the percent yield of the reaction.
3. Explain whether or not mass was conserved.
As we've been talking about, chemical reactions summarize chemical change and are represented by chemical equations. Reactants are on the left and products are on the right. Equations are balanced using coefficients to ensure that mass is conserved (we can't break that law, y'all). State symbols are used to denote the state of matter each reactant and product are in at the time of reaction.
Reminder: (s) = solid (l) = liquid (g) = gaseous (aq) = aqueous (dissolved in water)
Having said all that, let's take a look at some chemical reactions. You need to predict the results, measure the amount of reactants that you used and determine how much product you should have obtained. Here is the writeup.
The balanced chemical equation represents what is theoretically possible when a reaction is carried out under ideal conditions. It allows for the expected amount of products to be calculated. This is called the theoretical yield (TY). The objective is to get as close to the TY as possible. But we don't live in a perfect world and many factors, especially in large-scale processes, result in a reduced yield of products. Page 23 in your text lists some factors. This reduced yield is called either the experimental yield or actual yield (AY). You need these two values to determine the percentage yield of the actual process. The formula is below.
Percent error determines the precision of your experiment. The formula is below. You want your percent error to be as close to 0.00% as possible, as that means you are close to the accepted value for the experiment.
You will be expected to perform these calculations with every experiment.
Mission 3: Part vs. Whole
Mission Objectives. You should be able to...
1. Explain what percent composition means in terms of a compound.
2. Calculate percent composition.
Tyler DeWitt explains percent composition. This determines how much of each element is in a compound. Afterward the video, we will practice. Be sure you still have your handouts from last week.
Mission Objectives. You should be able to...
1. Explain what percent composition means in terms of a compound.
2. Calculate percent composition.
Tyler DeWitt explains percent composition. This determines how much of each element is in a compound. Afterward the video, we will practice. Be sure you still have your handouts from last week.
Mission 4: Empirical vs Molecular Formulas
Mission Objectives. You should be able to...
1. Distinguish between empirical formulas and molecular formulas.
2. Determine the empirical formula of a compound.
3. Determine the molecular formula of a compound.
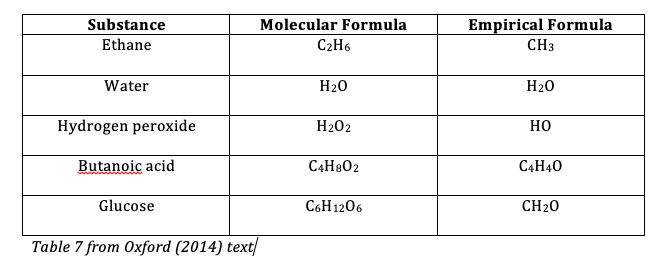
Tyler DeWitt explains empirical and molecular formulas. An empirical formula (EF) of a compound is the simplest whole-number ratio of atoms or amount (in mol) of each element present in a compound. The molecular formula (MF) is the actual number of atoms or amount (in mol) of elements in one structural unit or one mole of the compound, respectively. Sometimes the EF is the same as the MF. See the table below.
Mission Objectives. You should be able to...
1. Distinguish between empirical formulas and molecular formulas.
2. Determine the empirical formula of a compound.
3. Determine the molecular formula of a compound.
Tyler DeWitt explains empirical and molecular formulas. An empirical formula (EF) of a compound is the simplest whole-number ratio of atoms or amount (in mol) of each element present in a compound. The molecular formula (MF) is the actual number of atoms or amount (in mol) of elements in one structural unit or one mole of the compound, respectively. Sometimes the EF is the same as the MF. See the table below.
Mission 5: Leftovers
Mission Objectives. You should be able to...
1. Define "limiting reactant" and "excess reactant."
2. Determine the limiting reactant and the excess reactant in an experiment.
3. Solve limiting reactant problems.
Limiting Reactants. Chemical reactions should theoretically go to completion, but they do not because some reactants are used up faster than others. The reactant that runs out first (the limiting reactant or limiting reagent: LR) determines the amount of product that is formed. For example, if you're making cheese sandwiches and you have 16 pieces of bread and five pieces of cheese, but the recipe calls for two pieces of bread and one piece of cheese...how many sandwiches can you make before you run out of ingredients? Which is the limiting ingredient? Which is the excess ingredient (the reactant that is left over: ER)?
In order to complete LR/ER problems, you need to remember how to read a balanced chemical equation and determine the correct mole ratios. Basically, the mole ratio is key in determining how much product is obtained.
Having said that, here's a quick-N-easy set of guidelines on solving LR problems.
Here's an IB-level video to give you an idea of the math that is involved in understanding the concept. Don't go crazy over doing the problems; we will work those out in class. Just try to understand the concept and get familiar with how to do the work.
Mission Objectives. You should be able to...
1. Define "limiting reactant" and "excess reactant."
2. Determine the limiting reactant and the excess reactant in an experiment.
3. Solve limiting reactant problems.
Limiting Reactants. Chemical reactions should theoretically go to completion, but they do not because some reactants are used up faster than others. The reactant that runs out first (the limiting reactant or limiting reagent: LR) determines the amount of product that is formed. For example, if you're making cheese sandwiches and you have 16 pieces of bread and five pieces of cheese, but the recipe calls for two pieces of bread and one piece of cheese...how many sandwiches can you make before you run out of ingredients? Which is the limiting ingredient? Which is the excess ingredient (the reactant that is left over: ER)?
In order to complete LR/ER problems, you need to remember how to read a balanced chemical equation and determine the correct mole ratios. Basically, the mole ratio is key in determining how much product is obtained.
Having said that, here's a quick-N-easy set of guidelines on solving LR problems.
Here's an IB-level video to give you an idea of the math that is involved in understanding the concept. Don't go crazy over doing the problems; we will work those out in class. Just try to understand the concept and get familiar with how to do the work.