C8: Photovoltaic & Dye-Sensitized Solar Cells
Conjugation is the interaction of alternating double bonds, for example, in organic molecules, to produce a delocalized array of pi electrons all over the atoms. Molecules with conjugated bonds can absorb visible light, with longer conjugated systems absorbing light of longer wavelength. All the carbon atoms in such systems have sp2 hybridization: the pi-electron clouds of adjacent double bonds overlap with one another and form a large cloud of delocalized electrons. This type of multi-center chemical bonding is known as electron conjugation. It is similar to the electron delocalization seen in benzene and produces a chain of carbon-carbon bonds with a bond order of 1.5.
Looking at the examples on page 711, those compounds with alternating double and single bonds have conjugation. A and B show conjugation but C and D do not. For conjugated alkenes, the higher the degree of conjugation, the longer the wavelength of light that can be absorbed.
Looking at the examples on page 711, those compounds with alternating double and single bonds have conjugation. A and B show conjugation but C and D do not. For conjugated alkenes, the higher the degree of conjugation, the longer the wavelength of light that can be absorbed.
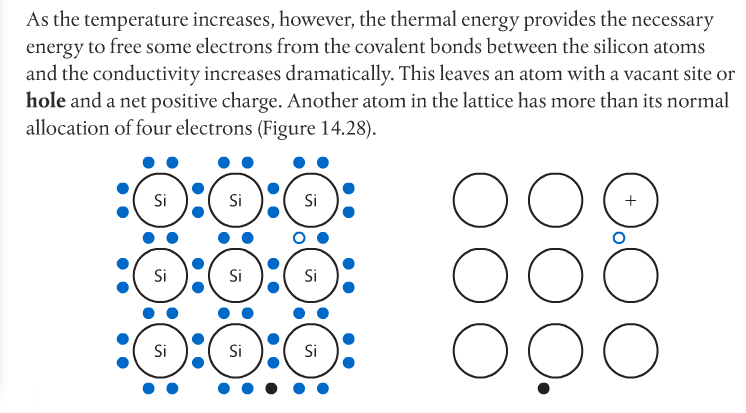
Semiconductors have electrical conductivity midway between that of conductors and insulators. The conductivity of a semiconductor increases with temperature, in contrast to that of conductors. Conductors are typically metals with low ionization energies and therefore, freely moving electrons.
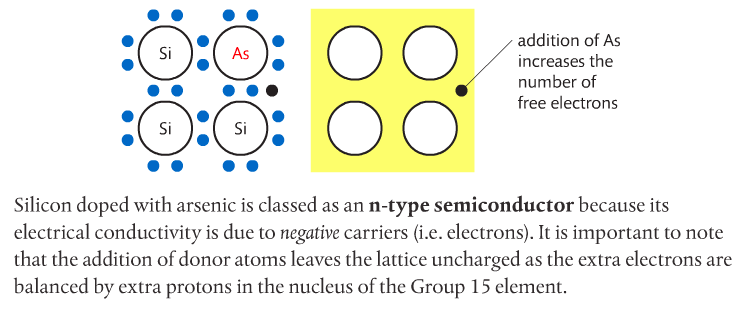
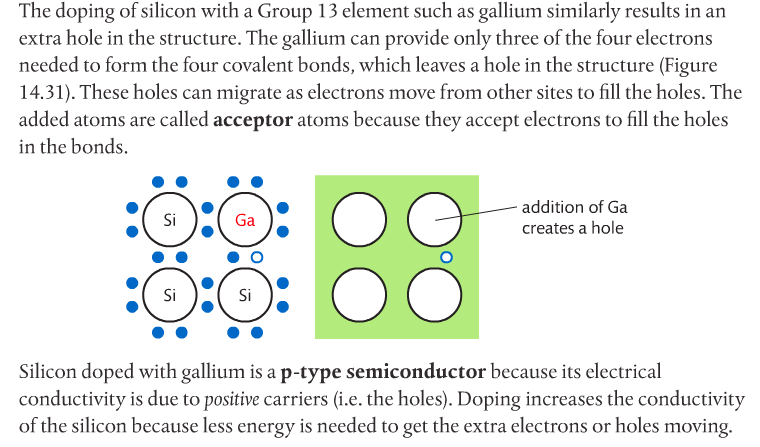
Photovoltaic cells made of semiconductors can absorb photons of light, resulting in electrons being knocked free from atoms and creating a potential difference. Semiconductor materials for such cells are often Group 14 elements such as silicon and germanium. Conductivity can be increased by doping the semiconductor with impurities from Group 15 elements such as phosphorous to create n-type semiconductors, or Group 13 elements such as boron to create p-type semiconductors. Page 712-713 go into detail about this. The below images come from the Pearson textbook.
Photovoltaic cells made of semiconductors can absorb photons of light, resulting in electrons being knocked free from atoms and creating a potential difference. Semiconductor materials for such cells are often Group 14 elements such as silicon and germanium. Conductivity can be increased by doping the semiconductor with impurities from Group 15 elements such as phosphorous to create n-type semiconductors, or Group 13 elements such as boron to create p-type semiconductors. Page 712-713 go into detail about this. The below images come from the Pearson textbook.
Photovoltaic cells absorbs photons in a semiconducting material, which causes some valence electrons to be removed, resulting in some ionization in the cell. A charge separation occurs in the semiconductor which allows for a one-way flow of electrons. The cell can then be linked to an external circuit where the flow of electrons provides electrical power.
In a dye-sensitized solar cell (DSSC), photons are absorbed by a dye in a way similar to the absorption of photons by chlorophyll in photosynthesis. Electrons in the dye are then injected into a titanium (IV) oxide nanoparticle layer, which conducts the electrons to the anode. Once a dye molecule as emitted its excited electron, it needs to gain another electron. To do this, dye-coated titanium (IV) oxide nanoparticles are immersed in a solution of I- ions. The I- ions release electrons to the dye on the titanium (IV) oxide, becoming oxidized to tri-iodide ions. They can accept electrons at the cathode, being reduced back to iodide ions. See page 714, Figure 7.
C8 PowerPoint
In a dye-sensitized solar cell (DSSC), photons are absorbed by a dye in a way similar to the absorption of photons by chlorophyll in photosynthesis. Electrons in the dye are then injected into a titanium (IV) oxide nanoparticle layer, which conducts the electrons to the anode. Once a dye molecule as emitted its excited electron, it needs to gain another electron. To do this, dye-coated titanium (IV) oxide nanoparticles are immersed in a solution of I- ions. The I- ions release electrons to the dye on the titanium (IV) oxide, becoming oxidized to tri-iodide ions. They can accept electrons at the cathode, being reduced back to iodide ions. See page 714, Figure 7.
C8 PowerPoint
| clara_option_c8.pptx |

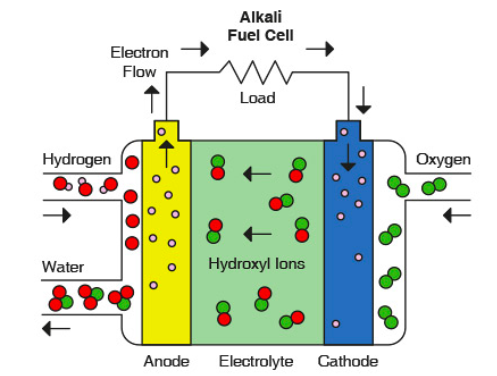
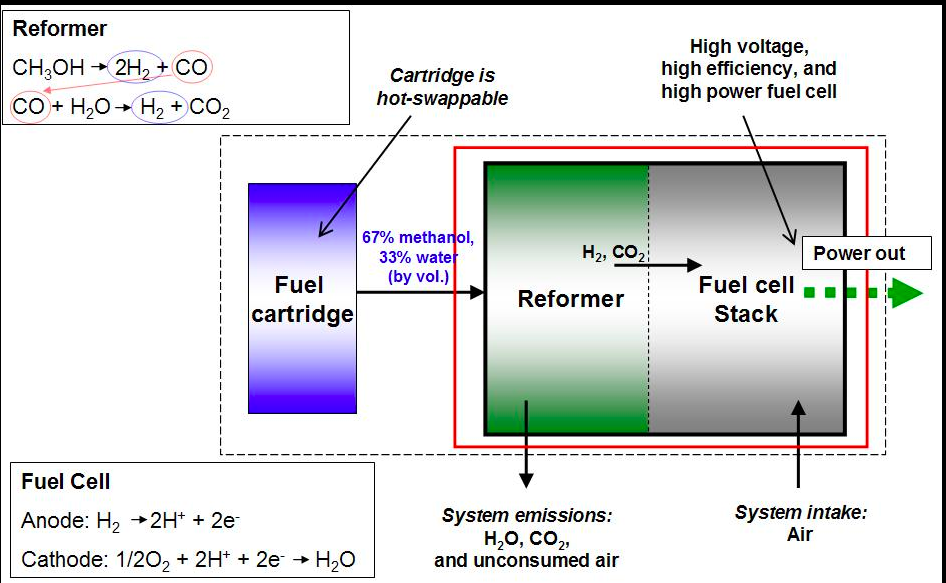
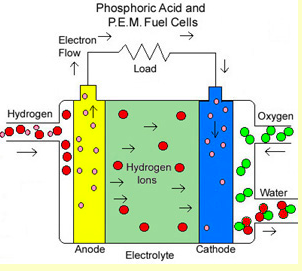
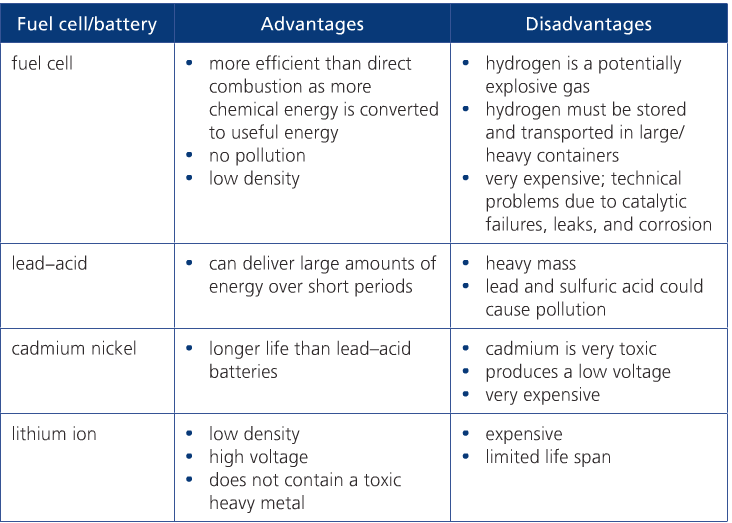



 RSS Feed
RSS Feed