Redox Processes I
Content Review: Oxidation & Reduction Batteries
Mission 1: RED-OX!!!
Mission Objectives. You should be able to:
1. Describe redox reaction in terms of electrons lost and gained.
2. Explain oxidation number and know the rules for determining oxidation numbers.
3. Contrast oxidizing agent and reducing agent.
4. Deduce the oxidation state of an atom or ion in a compound.
5. Identify species that are oxidized, species that are reduced, and spectator ions.
The definitions of oxidation and reduction have evolved over the years. They used to mean loss of oxygen/gain of oxygen. However, the definitions have become broader and we now refer to them as loss or gain of electrons. The reason for this is because many chemical processes do not include oxygen, but do undergo oxidation and reduction.
Handy little reference chart can be found HERE.
Oxidation and reduction are complementary processes. Oxidation refers specifically to the loss of electrons. Reduction refers to the gain of electrons. Transferring electrons from one substance to another leads to a flow of electrons, which is nothing but an electric current. Reversing the process (using electricity to drive redox reactions) allows for stable compounds to decompose into their component ions and elements. This process is called electrolysis. Applications of redox reactions are significant because this is the foundation for electrochemistry. Think: batteries. What would happen if there were no batteries?
Mission 1: RED-OX!!!
Mission Objectives. You should be able to:
1. Describe redox reaction in terms of electrons lost and gained.
2. Explain oxidation number and know the rules for determining oxidation numbers.
3. Contrast oxidizing agent and reducing agent.
4. Deduce the oxidation state of an atom or ion in a compound.
5. Identify species that are oxidized, species that are reduced, and spectator ions.
The definitions of oxidation and reduction have evolved over the years. They used to mean loss of oxygen/gain of oxygen. However, the definitions have become broader and we now refer to them as loss or gain of electrons. The reason for this is because many chemical processes do not include oxygen, but do undergo oxidation and reduction.
Handy little reference chart can be found HERE.
Oxidation and reduction are complementary processes. Oxidation refers specifically to the loss of electrons. Reduction refers to the gain of electrons. Transferring electrons from one substance to another leads to a flow of electrons, which is nothing but an electric current. Reversing the process (using electricity to drive redox reactions) allows for stable compounds to decompose into their component ions and elements. This process is called electrolysis. Applications of redox reactions are significant because this is the foundation for electrochemistry. Think: batteries. What would happen if there were no batteries?
Mission 2: Break it in HALF!!!
Mission Objectives. You should be able to:
1. Write redox half-equations.
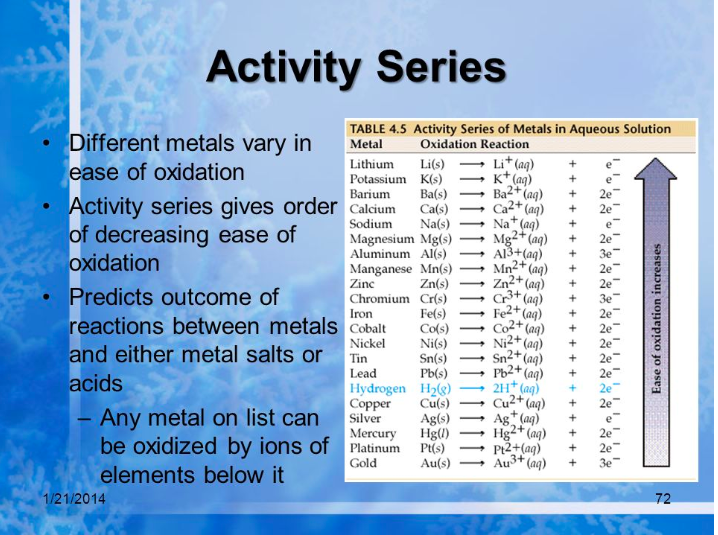
Let's recall the activity series of metals. It is a list of elements in order of reactivity. Metals higher on the activity series can replace metals lower than them. Below you'll see a series of half-equations and their order of reactivity, which is opposite their ease of oxidation. You have a similar chart in your Data Booklet.
Mission Objectives. You should be able to:
1. Write redox half-equations.
Let's recall the activity series of metals. It is a list of elements in order of reactivity. Metals higher on the activity series can replace metals lower than them. Below you'll see a series of half-equations and their order of reactivity, which is opposite their ease of oxidation. You have a similar chart in your Data Booklet.
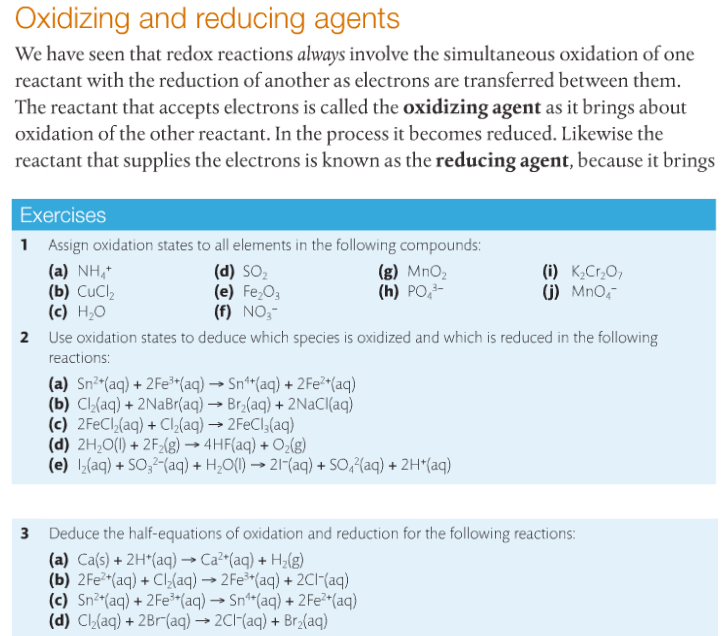
We will practice writing half-equations with questions 2 & 3 below before we get into acidic and/or neutral solutions. The steps on writing full redox equations are found below AND on page 217-18 in your text. We will work through #4 together in class.
Oxidizing & Reducing Agents. Oxidizing agents cause another species to be oxidized and is itself reduced in the process. Reducing agents cause another species to be reduced and is itself oxidized in the process. Let's learn more about them.
Mission 3: The Winkler Method
Mission Objectives. You should be able to...
1. Use the Winkler Method to calculate BOD.
Mission Objectives. You should be able to...
1. Use the Winkler Method to calculate BOD.