Macromolecules
Organic chemistry is the study of organic (carbon-containing) compounds. Carbon is an essential element in almost all biological molecules, which is why scientists refer to life on Earth as being carbon-based.
Carbon is in group 14 of the periodic table, which means that it has four valence electrons in its outermost energy level. This means that carbon can form four covalent bonds with other atoms. This results in the formation of a variety of organic compounds. They are in the shapes of chains, both straight and branched, and rings.
Macromolecules are large molecules that are formed from the joining of smaller organic molecules. They are sometimes called polymers, which are molecules formed from repeating units of identical compounds called monomers. There are four kinds of macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
Carbon is in group 14 of the periodic table, which means that it has four valence electrons in its outermost energy level. This means that carbon can form four covalent bonds with other atoms. This results in the formation of a variety of organic compounds. They are in the shapes of chains, both straight and branched, and rings.
Macromolecules are large molecules that are formed from the joining of smaller organic molecules. They are sometimes called polymers, which are molecules formed from repeating units of identical compounds called monomers. There are four kinds of macromolecules: carbohydrates, lipids, proteins, and nucleic acids.
|
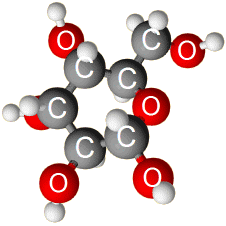
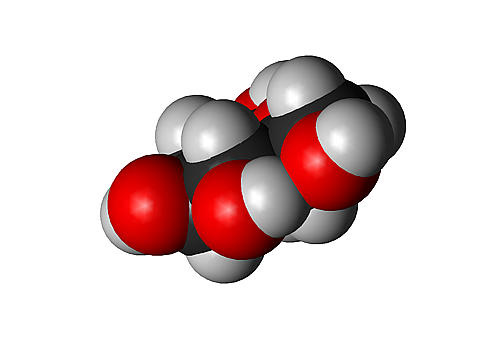
Carbohydrates. Carbs are compounds composed of C H and O atoms in a particular arrangement. The arrangement is this: for every carbon, there is one oxygen and two hydrogen. The generic formula is (CH2O)n. “n” means the number of units in the chain. Chains that have values of n that range from 3 to 7 are called monosaccharaides (simple sugars). Glucose is a monosaccharide.
When monosaccharides are linked, they form larger molecules called polysaccharides. Disaccharides are two monosaccharides that have been linked. Lactose and sucrose are disaccharides, which are also energy sources. Glycogen is a polysaccharide that is stored glucose found in liver and muscle tissue. Cellulose is found in plants and provides structural support. Chitin is a polysaccharide that contains nitrogen and forms the exoskeleton of some shellfish and insects. Images courtesy of www.abcbodybuilding.com and BBC GCSE. |
|
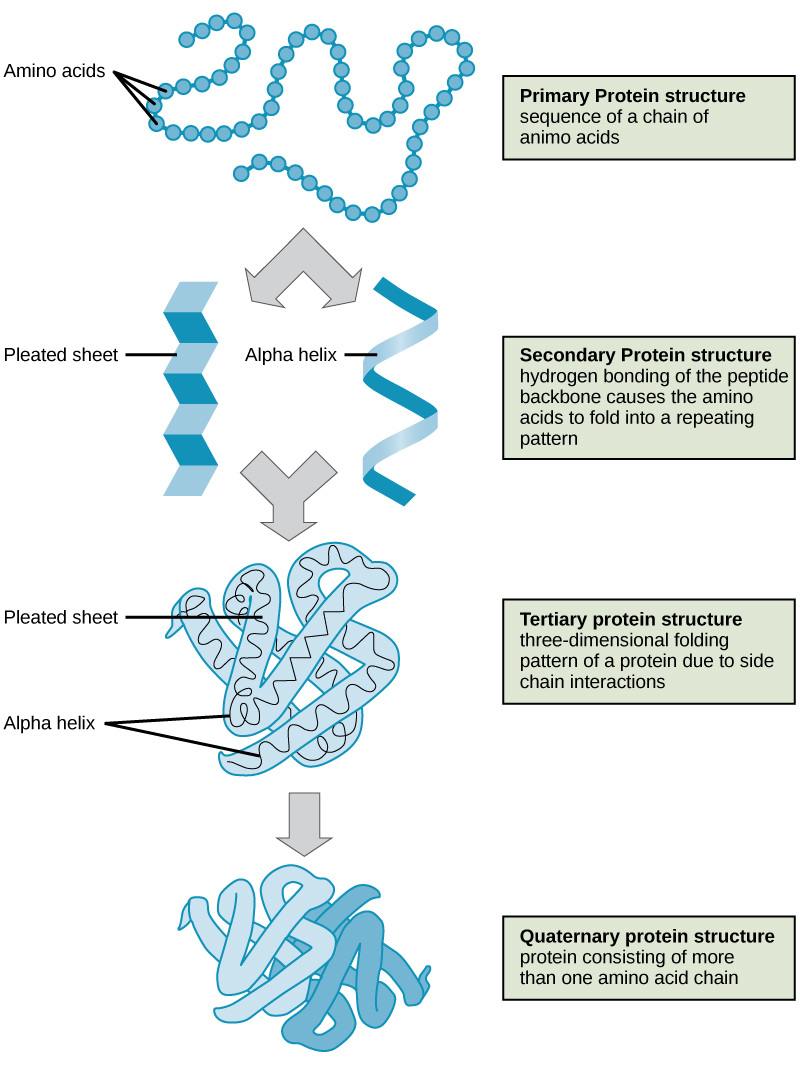
Proteins. Compounds made of amino acids are called proteins. Amino acids are small compounds made up of C, H, O, N, and sometimes S. They have the same general structure. There is a central carbon atom that has one H attached. The other three bonds are with an amino group (-NH2), a carboxyl group (-COOH), and a variable group (-R). The “-R” is what makes each amino acid different. There are 20 different variable groups, which yield 20 amino acids, and proteins are made up of different combinations of all 20 different amino acids. Covalent bonds called peptide bonds join aminos together to make proteins. The bond specifically forms between the amino group and the carboxyl group (-NH2 and -COOH).
Proteins make up about 20% of your body mass and are involved in nearly every bodily function. Image courtesy of archive.cnx.org |
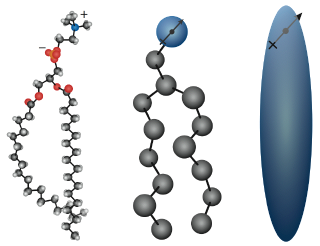
Lipids. Lipids are fats, waxes, and oils made up of carbon and hydrogen. They are composed of fatty acids, glycerol and other components. The primary function of a lipid is to store energy. Triglycerides are lipids that are fats if they’re solid at room temperature, and oils if they’re liquid at room temperature. They are stored in the fat cells of the body. Plants are coated in wax to prevent water loss.
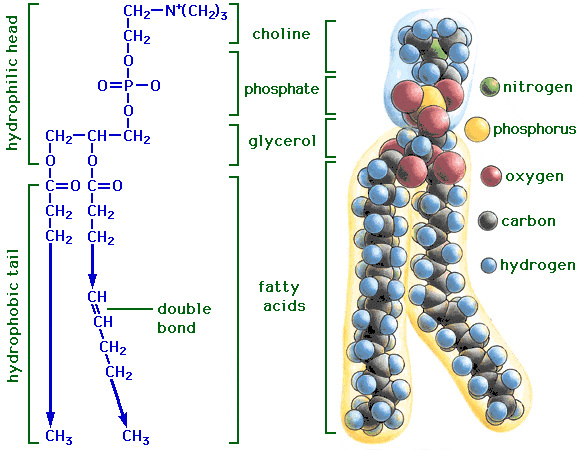
The basic structure of a lipid includes a fatty acid tail .The tail is a chain of carbon atoms bonded to hydrogen and another carbon. Tails with single C-C bonds are saturated fats. If there is at least one C=C bond, then the tails are unsaturated fats. Phospholipids are special lipids that are responsible for the structure and function of the plasma membrane. Steroids are lipids that include substances such as hormones and cholesterol. Images courtesy of Gezelter Lab and medicalinf0.blogspot.com. |
|
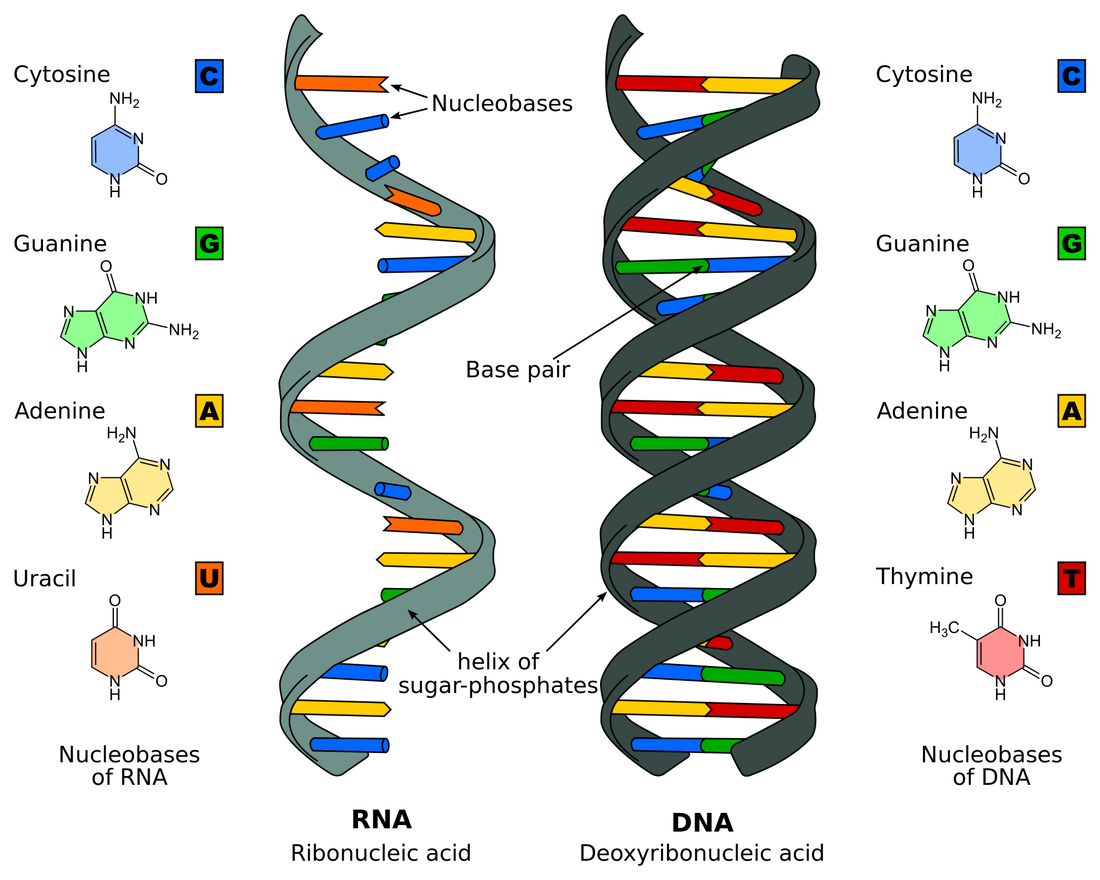
Nucleic acids. Nucleic acids are complex macromolecules that are involved in storing and transmitting genetic information. They are made up of small repeating units composed of C, N, O, P, and H atoms, called nucleotides. There are six nucleotides, all of which contain a phosphate, a nitrogen base, and a ribose sugar.
There are two types of nucleic acid: DNA and RNA. They both have a sugar of one nucleotide bonding to the phosphate of another. A nucleotide with three phosphate groups is ATP, which is stored chemical energy that is used by cells in different reactions. Energy is released when the bond between the second and third phosphate group is broken. Less energy is released when the bond between the first and second phosphate group is broken. |