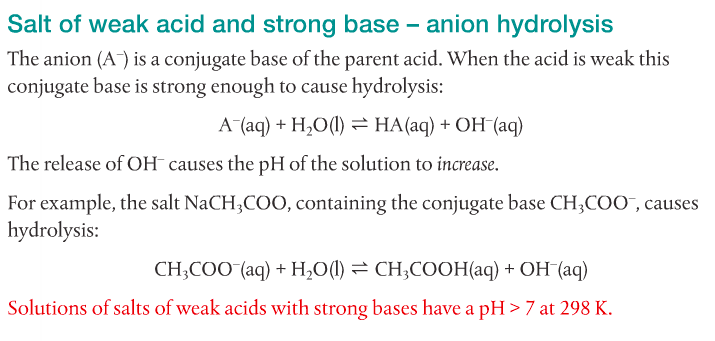Buffers, Indicators & Salt Hydrolysis
Mission Objectives. You should be able to...
1. Sketch and identify the graphs of pH against volume for titrations involving strong and weak acids and bases with an explanation of their important features.
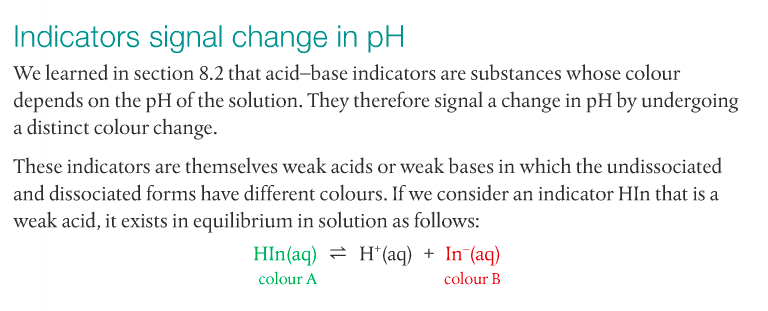
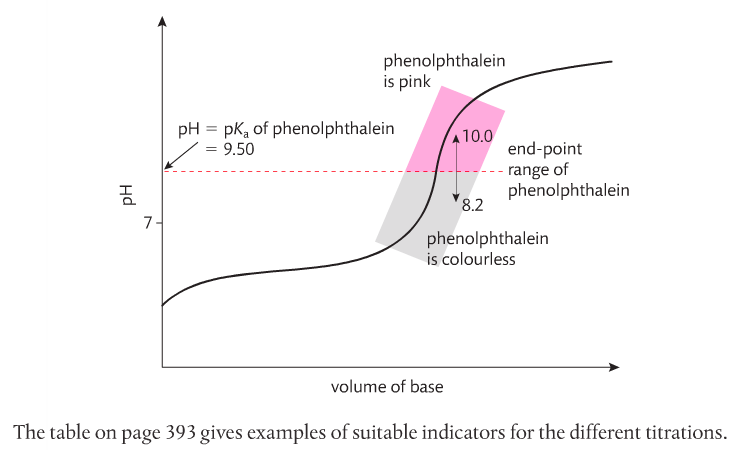
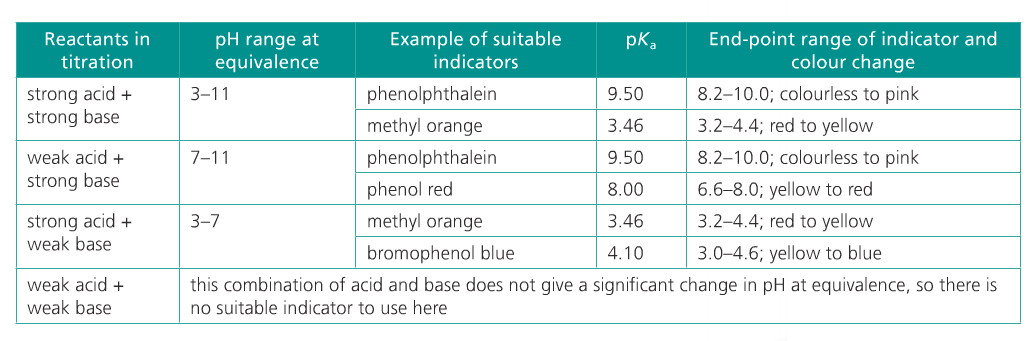
2. Select an appropriate indicator for a titration, given the equivalence point of the titration and the end point of the indicator.
3. Describe how buffer solutions can be prepared by either mixing a weak acid/base with a solution of a salt containing its conjugate, or by partial neutralization of a weak acid/base with a strong acid/base.
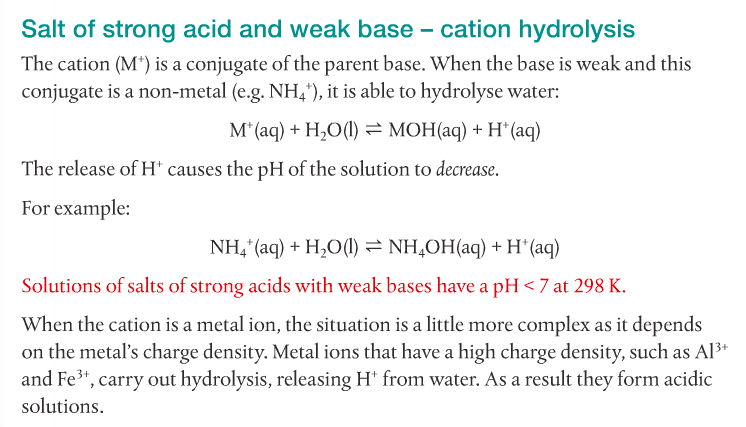
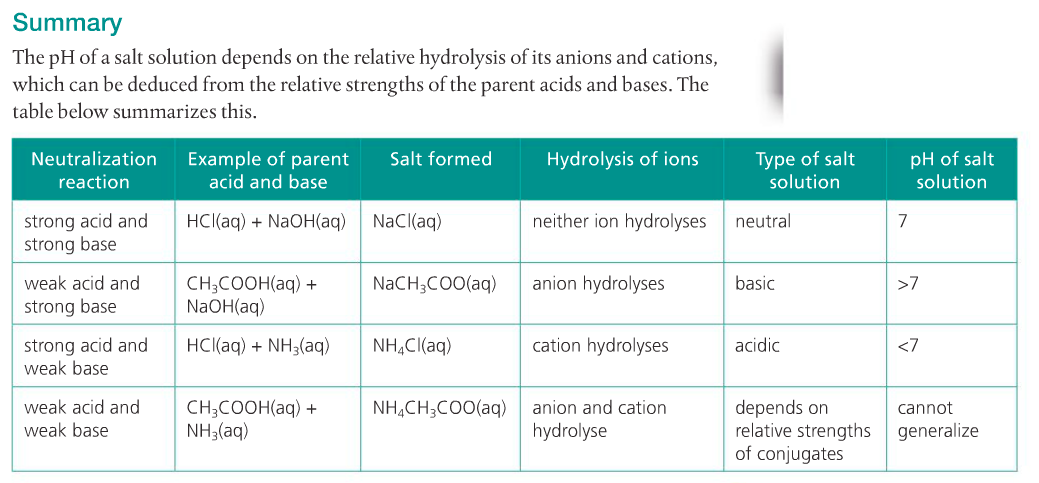
4. Predict the relative pH of aqueous salt solutions formed by the different combinations of strong and weak acids and bases.
The below content was taken from the 2016 HL Pearson text.
1. Sketch and identify the graphs of pH against volume for titrations involving strong and weak acids and bases with an explanation of their important features.
2. Select an appropriate indicator for a titration, given the equivalence point of the titration and the end point of the indicator.
3. Describe how buffer solutions can be prepared by either mixing a weak acid/base with a solution of a salt containing its conjugate, or by partial neutralization of a weak acid/base with a strong acid/base.
4. Predict the relative pH of aqueous salt solutions formed by the different combinations of strong and weak acids and bases.
The below content was taken from the 2016 HL Pearson text.