Housekeeping: Happy New Year, guys! We will be doing some solution chemistry for the next couple of weeks and then move into some basic physics. Solution chemistry is very easy.
Agenda:
1. Intro to solution chemistry
2. The solvation process
Content Review:
Download these notes: Solutions Solubility
Student Missions:
Mission 1: The Problem is the Solution
Mission Objectives. You should be able to:
1. Define these terms: solution, solute, solvent, solubility, soluble, insoluble, miscible, and immiscible.
2. Provide examples of solutions in all three states of matter.
3. Calculate solution concentrations using percent by mass and percent by volume.
Image from slideplayer.com
Agenda:
1. Intro to solution chemistry
2. The solvation process
Content Review:
Download these notes: Solutions Solubility
Student Missions:
Mission 1: The Problem is the Solution
Mission Objectives. You should be able to:
1. Define these terms: solution, solute, solvent, solubility, soluble, insoluble, miscible, and immiscible.
2. Provide examples of solutions in all three states of matter.
3. Calculate solution concentrations using percent by mass and percent by volume.
Image from slideplayer.com
For the following video, start at 2:15 where he begins discussing solutions.
Calculating percent by mass and percent by volume. Watch the following video. Stop at 5:00. Make sure you take notes and understand the formulas. Complete the practice problems below the video.
Practice Problems
Mission 2: SOLVATION, Y'ALL!!!
Mission Objectives. You should be able to:
1. Describe the process of solvation.
2. Compare and contrast the solvation processes between ionic compounds and covalent compounds.
3. Identify the factors that affect solvation.
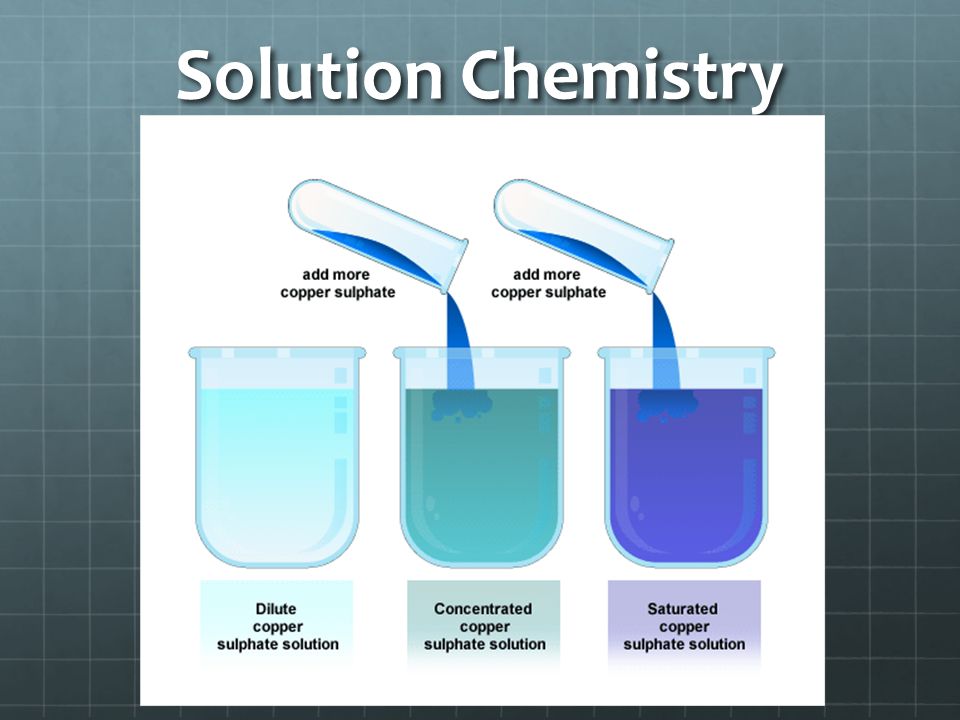
4. Define and explain the following terms: unsaturated, saturated, supersaturated, concentrated and dilute.
Mission 2: SOLVATION, Y'ALL!!!
Mission Objectives. You should be able to:
1. Describe the process of solvation.
2. Compare and contrast the solvation processes between ionic compounds and covalent compounds.
3. Identify the factors that affect solvation.
4. Define and explain the following terms: unsaturated, saturated, supersaturated, concentrated and dilute.
Here is a marvelous powerpoint that talks about the solvation process and solubility.
Here is the lab we will do in class.
Here is the lab we will do in class.
| grade_8_solutions_lab.docx |

 RSS Feed
RSS Feed