Click HERE for Missions.
|
Housekeeping: We are now investigating chemical reactions and the beginning of stoichiometry, which is in Chapter 1 of your text.
Click HERE for Missions.
0 Comments
Housekeeping: We are now investigating chemical bonding in the elements. This corresponds to Chapters 4 and 14 in the text. Hopefully you are keeping up with the readings in your text and on the website.
Content Review: Links: Chemical Bonding This page contains PowerPoints that cover ionic and covalent bonding and a summary table. This is a PowerPoint on Chemical Names & Formulas. Click HERE for Missions. Housekeeping: We are now studying periodicity, which is Chapter 3 in your text. Your exam over Chapter 2 is scheduled for next Wednesday, August 29. Periodicity takes about a week, content-wise.
For HL, I decided to hold off on Chapter 12 until we finish Chapter 3, as the content is more directly connected to Chapter 3 than Chapter 2. You'll see. CLICK HERE FOR MISSIONS Housekeeping: Welcome back to school! For those of you new to BIS, I'm Dr. Holt, the Mad!Scientist! I will show you how to use this website. Be sure to bookmark it for easy access. As far as content, we will start with Chapter 2: Atomic Structure. We will return to Chapter 1 after we finish Chapter 4. Content Review: Atomic Theory Structure Electron Behavior Electron Configuration Agenda: Housekeeping Unit 1: The Atom Missions Atomic Structure Atomic Theory Nuclear Chemistry Isotopes Electrons Electron Configuration Housekeeping: We are now working in Chapter 8: Acids & Bases. This will be the last chapter we cover for this year. You need to start seriously thinking about your IA topic. A huge chunk of next year will be devoted to you working on your IAs, but that's only if you decide on a topic now. The due date for the IA topics will be May 31. Your topic submission must include a RQ, hypothesis, and IV/DV/Controls. A failure to do this means a loss of time in the fall, which none of you can afford. Also, I will get on your last nerve reminding you of what you owe me. Once I have confirmed your topics, we will (as a class) come up with a schedule for IA progress. Once we have completed the course content (Chapters 9, 10 & 11 for SL; 9, 10, 11, 19, 20, & 21 for HL; and then Option C: Energy, [which I expect will take most of 1st semester), you will have time to work on your IAs both inside and outside of class. Content Review: Textbook: Chapter 8/18 Links: Acid-Base Videos Click HERE for the Missions. For the acid-base data lab, download the below file so that you can collect your data in an organized fashion.
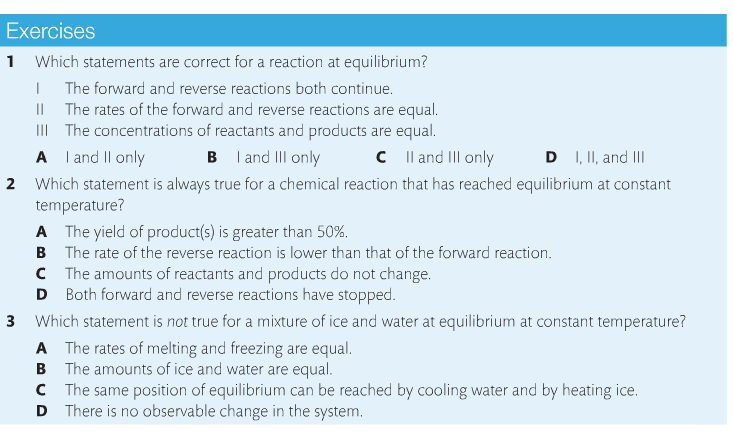
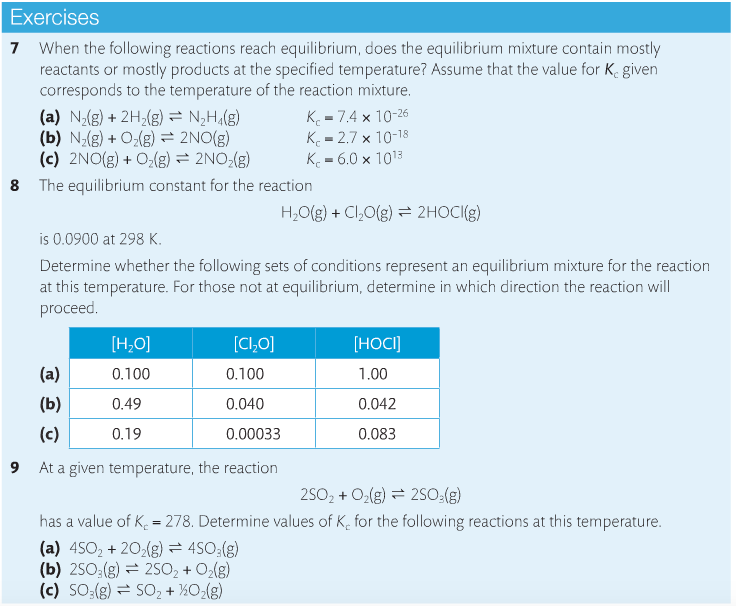
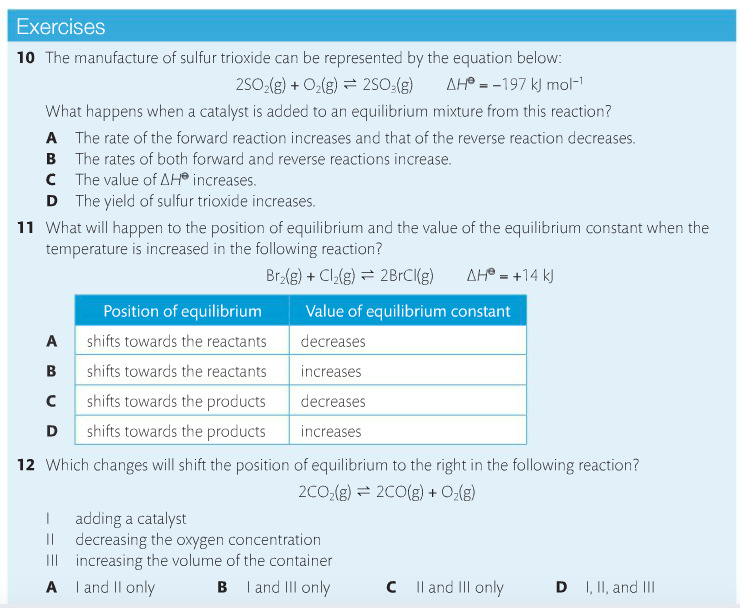
Housekeeping: We are now covering Chapter 7, Equilibrium. The exam for chapters 6 & 7 are on April 6 for SL and April 16 for HL Content Review: Textbook: Chapter 7 Links: IB Chemistry Home Notes Mission 1: Balance, Baby...Balance! Mission Objectives: You should be able to: 1. Describe characteristics of chemical and physical systems at equilibrium. 2. Deduce the equilibrium constant (Kc) from an equation. 3. Explain Le Chatelier's Principle in your own words. 4. Define and explain reaction quotient. 5. Explain what it means when reactions must shift either to the left or the right. 6. Describe the effects of changing concentration, temperature and pressure on reactions at equilibrium. 7. Describe what happens when a catalyst is added to a reaction at equilibrium. 8. Explain what the Haber Process and the Contact Process are, and their significance. Many chemical reactions are reversible and exist in a state of equilibrium. Equilibrium occurs when the forward and reverse reactions (designated by a double arrow <-->) occurring simultaneously with products and reactants being constantly changing into each other. Understanding the nature of equilibrium allows researchers to control reaction conditions and maximize product yield. This is significant in industry, as you'll soon learn. At equilibrium: (1) Forward and reverse reactions are occurring at equal rates, (2) There is no change in [reactants] and [products], (3) There is no change in macroscopic properties such as density and color, (4) The equilibrium can be approached from either the forward or reverse direction, (5), Equilibrium is dynamic, and (6) Any changes in reaction conditions can affect equilibrium. Read about the reaction quotient (Q) here. It's also in your text. You need to know the relationship between Kc and Q. It is important to understand the position of the equilibrium, which means determining whether reactants are favored or products are favored, and how to manipulate the position to maximize product yield and industry profitability. The law of chemical equilibrium states that at a given temperature, the ratio of the concentration of products (raised to the power of their molar coefficients) to the concentration of the reactants (also raised to the power of their molar coefficients) is a constant (see the below image). This constant is called the equilibrium constant, Kc. (Don't forget, this is on your quiz). Look at the example on page 182 and take a look at Bozeman Science's explanation of the concept. Page 183 shows you how to write equilibrium constant expressions. This is something you need to become very familiar with in the determination of Kc. Page 184 provides a summary table showing the relationships between reaction conditions and equilibrium position. You need to know this, period. Le Chatelier's Principle is a rule that is useful for predicting the effect that changing conditions have on the equilibrium position. "If a change is made to a system in equilibrium, the balance between the forward and reverse reactions will shift to offset this change and return the system to equilibrium" (Oxford, 2014). Watch the below videos to gain a better understanding of Le Chatelier's Principle. Factors that affect equilibrium: Change in concentration. When an increase in concentration of a reactant occurs and disrupts equilibrium, the rate of the forward reaction will increase. The reverse reaction does not change, so the rates are no longer equal. When equlibrium is re-established, the mixture will have new concentrations and the equlibrium will have shifted to the right to favor the products. Addition of reactant causes the system to respond by removing reactant to make more product, which favors the forward reaction. Change in pressure. Equilibrium involving gases will be affected by a change in pressure if the reaction involves a change in the number of molecules. If a reaction at equilibrium is subject to an increase in pressure, the system responds by favoring the side with the smaller number of molecules. A decrease in pressure will cause a shift to the side with the larger number of molecules. As long as the temperature remains the same, Kc will be unchanged. Change in temperature. Kc is temperature-sensitive, so it changes as temperature changes. Exothermic reactions (delta-H negative) release energy and endothermic reactions (delta-H positive) absorb energy. A negative delta-H means that the forward reaction is exothermic. If this reaction is subjected to a temperature decrease, the system responds by producing heat and favoring the forward reaction. Equilibrium shifts to to the right as a result. This means more product is formed. A new equilibrium is achieved and Kc changes. Increasing the temperature favors the reverse reaction and shifts equilibrium to the left, decreasing the value of Kc and reducing the amount of product formed. Adding catalysts. Catalysts speed up reactions by reducing Ea. The rate of both reactions will increase by the same factor, which has no effect on the equilibrium position. Adding a catalyst reduces the time it takes to achieve equilibrium. Pages 188-189 provide excellent summary tables of the effects of temperature and catalysts on equilibrium. Study them. The following video describes the Haber Process for ammonia production. You need to become familiar with this. The Haber Process is not listed in the SL chapter, but I'm going to insist that SL learns it anyway, as it makes problem solving a lot more useful if you know it. Here's a quick and easy tutorial on the Haber process. Below is the corresponding IB video by Richard Thornley, and he discusses both the Haber Process and the Contact Process, both which you need to know. Describe the effects of adjusting the temperature and pressure on the formation of ammonia. The Contact Process is the production of sulfuric acid. It is composed of three simple reactions: the combustion of sulfur to form sulfur dioxide, the oxidation of sulfur dioxide to sulfur trioxide, and the combination of sulfur trioxide with water to produce sulfuric acid. The overall rate depends on step 2; the oxidation of SO2 to SO3. Similar to the Haber process, one can predict the condition that will favor the formation of product. Let's practice!!!
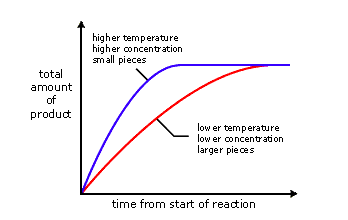
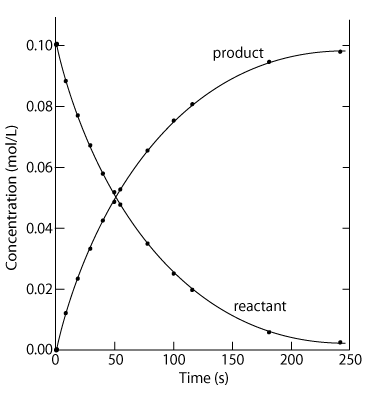
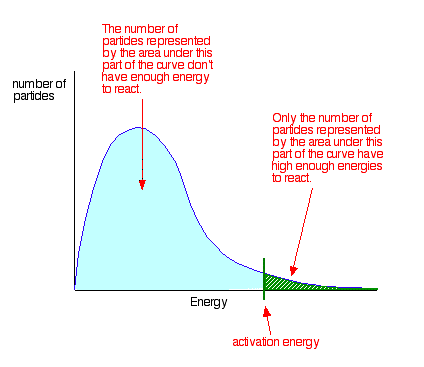
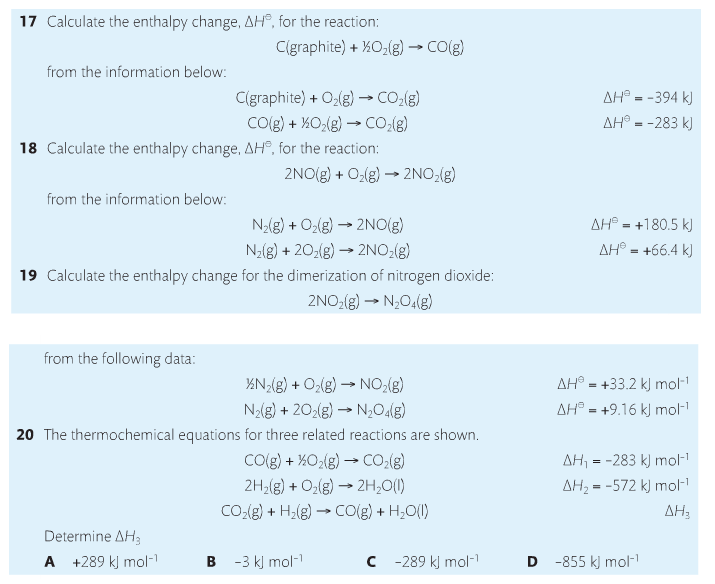
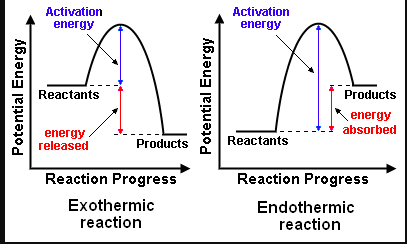
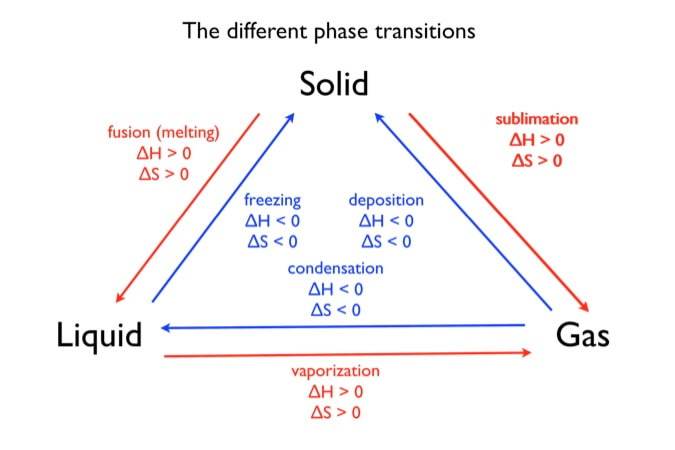
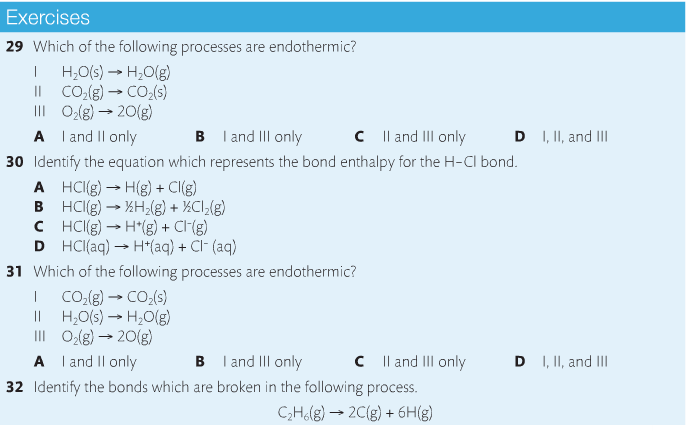
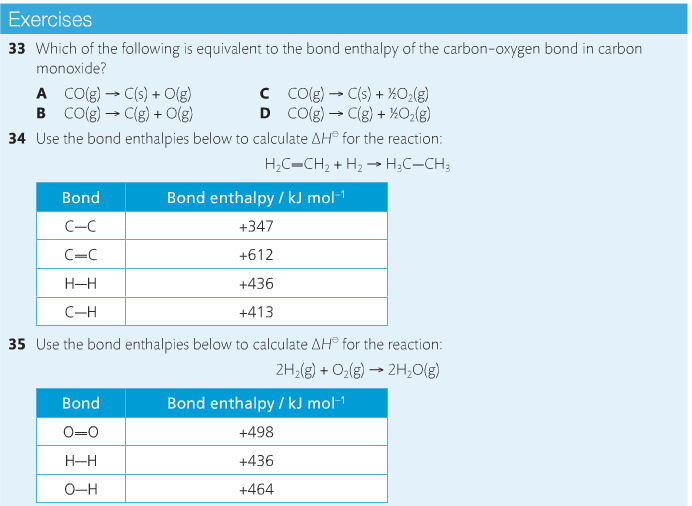
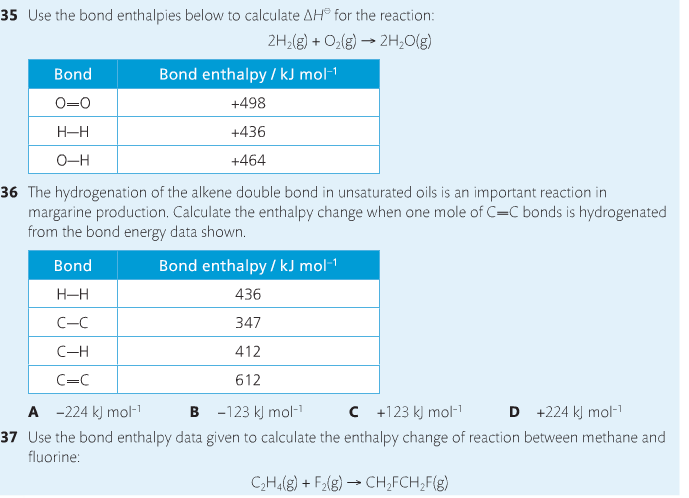
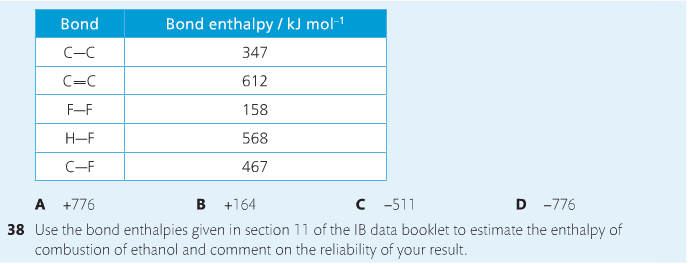
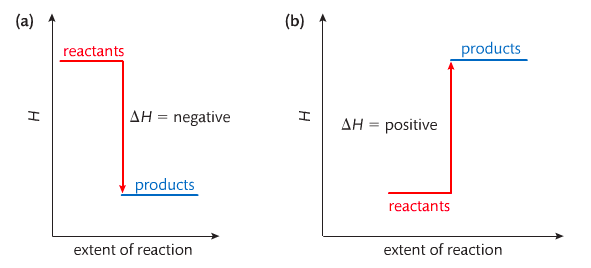
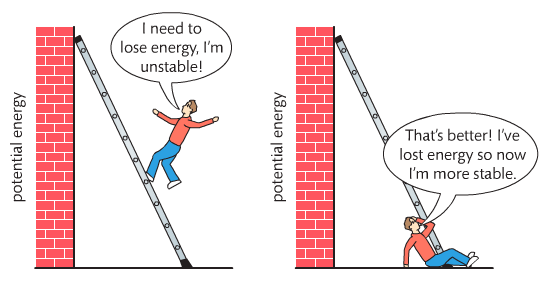
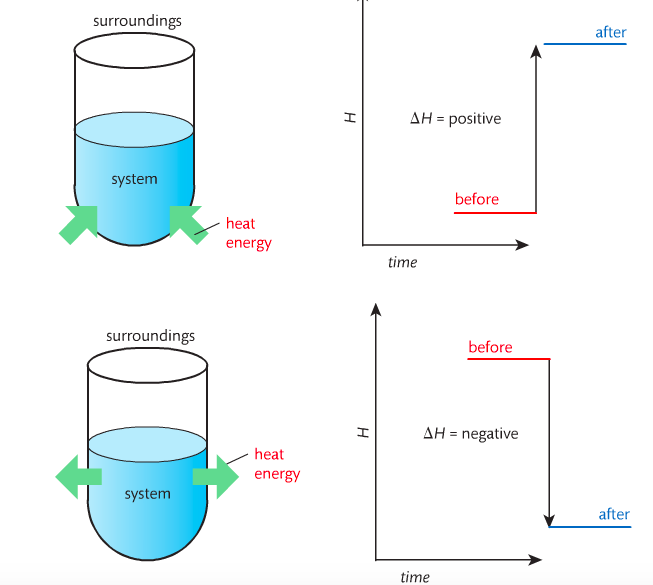
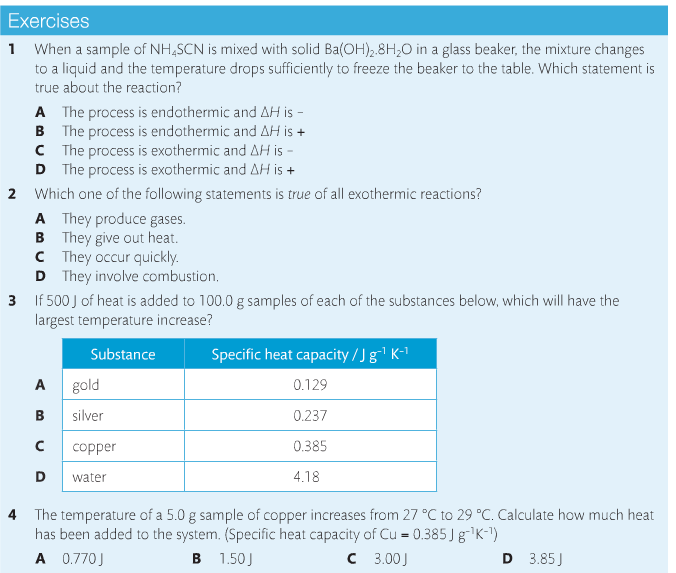
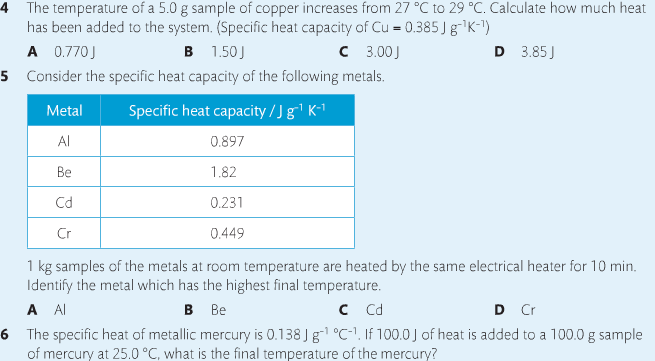
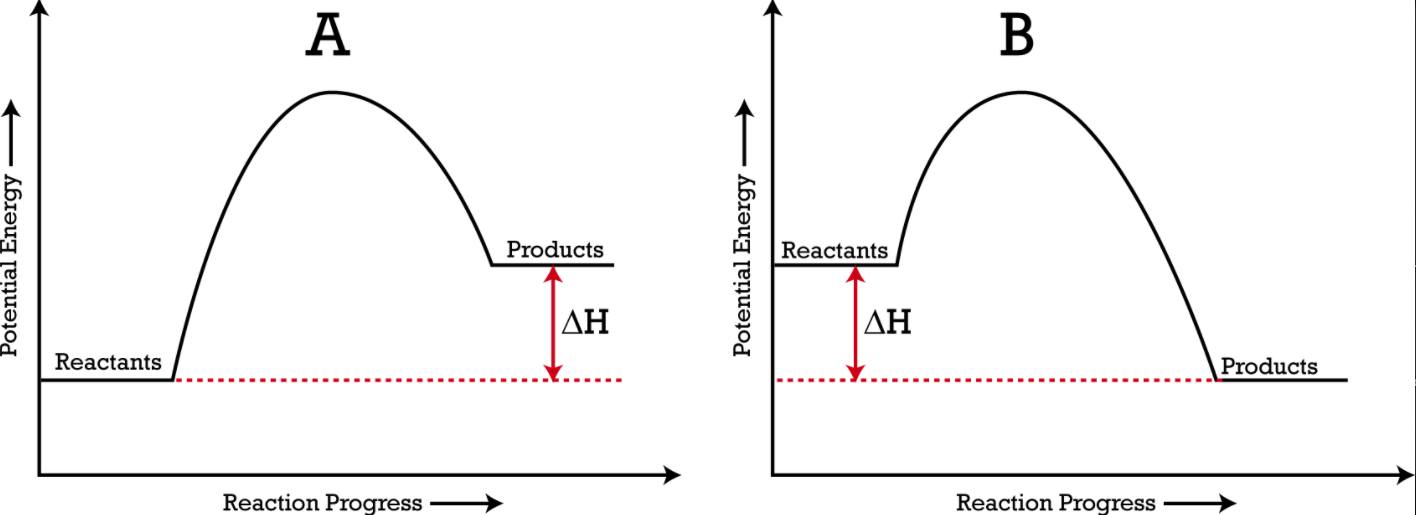
Housekeeping: Chapter 6 covers kinetics, which means "movement." In the context of chemistry, it refers to the movement of particles. We have covered the meat of kinetics, but now it is time for you to go back and study the bread. I have given you a set of three tasks that you must complete. I will provide links and videos to assist you with learning the material. I will combine chapter 6 with chapter 7, Equilibrium, as they are both very short chapters. Content Review Textbook: Chapter 6 Mad!Science! Links: Kinetics Agenda: Complete the tasks found here. You will need to reference this page. Mission 1: Reaction Mechanisms. Mission Objectives. You should be able to... 1. Explain the factors that affect rates of reaction. 2. Investigate rates of reaction experimentally and evaluate the results. The mechanism of a chemical reaction is the series of events that takes place as reactants are converted into products. You can read more about it on ChemWiki. Mission 2: Dem Graphs Again!!! Mission Objective. You should be able to... 1. Sketch and explain energy profiles with and without catalysts. Here is a simple graph from the BBC Bitesize website. What do you see? The point of Mission 2 is for you to be able to read and interpret different rate graphs and provide a reasonable explanation as to what is going on, so make sure you pay close attention to the graphs in your text. Mission 3: Dudes Named Max and Bolt (sort of)... Mission Objectives. You should be able to... 1. Construct a Maxwell-Boltzmann energy distribution curve to account for the probability of successful collisions and factors that affect them, including the effect of a catalyst. The Maxwell-Boltzmann graph is a distribution curve that shows the distribution of particles in a reaction. Below is a picture from chemguide.edu and a quick YouTube video summary. Study.com has a nice, extensive explanation that will help you answer the questions on the sheet. Copy and paste this link to listen and/or read: http://study.com/academy/lesson/the-boltzmann-distribution-temperature-and-kinetic-energy-of-gases.html Mission 1: Hess' Law Mission Objectives. You should be able to... 1. Complete enthalpy calculations using Hess' Law. Hess' Law is an application of the conservation of energy law. A chemical equation shows the net reaction; it is a summary of a number of different reactions which are added together and result in an overall reaction. We will spend time working on questions in class. Mission 2: Bond Enthalpy. Mission Objectives. You should be able to... 1. Calculate the enthalpy changes from known bond enthalpy and comparison of these with experimentally measured values. 2. Sketch and evaluate potential energy profiles in determining whether reactants or products are more stable and if the reaction is endothermic or exothermic. 3. Discuss the bond strength in ozone relative to oxygen and its importance to the atmosphere. Mr. Thornley is on the case. You will want to take notes from this video. He lays it out all clear and proper-like. Image courtesy of www.socratic.org. Bond breaking is an endothermic process. Energy must be absorbed to break the bonds between atoms and molecules. Bond making, by comparison, is an exothermic process. Energy is released when bonds are formed. Each bond type has its own energy requirements. Page 11 in your Data Booklet has a list of common bonds and their associated bond enthalpies. Recall that delta H for exothermic processes is negative and positive for endothermic processes. You need to be able to complete energy profiles for endothermic and exothermic reactions. Phase changes that are endothermic: melting, boiling and sublimation. Phase changes that are exothermic: condensation, freezing and deposition. Read more about this here. When looking at thermochemical equations, take note of the phase changes as reactants turn into products. That will help you determine whether the reaction is endothermic or exothermic. Housekeeping: Happy New Year, guys! We will be studying Chapter 5: Energetics & Thermochemistry this month. Agenda: 1. Heat vs Temperature 2. Enthalpy 3. Specific Heat 4. Hess' Law 5. Bond Enthalpy Content Review: Text: Chapter 5 Links: The Physics Classroom The Nature of Energy Measuring Energy Changes Student Missions: Mission 1: Energy. Some Days Are Better Than Others. Mission Objectives. You should be able to: 1. Explain how heat is a form of energy 2. Describe temperature as a measurement of kinetic energy 3. Explain the conservation of energy in chemical reactions 4. Compare endothermic and exothermic reactions 5. Explain the concept of system and surroundings Some things you should know. Temperature and heat are not the same thing. Heat is energy that results from a temperature change and produces an increase in disorder in how particles behave. Heat increases the average kinetic energy of molecules, which is measured by temperature. Professor Dave, aka Chemistry Jesus, explains it all. Chemical reactions are accompanied by energy changes. Bonds are broken and formed in reactions, which correspond to an increase or decrease in energy, Energy is the ability to do work or move an object against an opposing force. Examples of energy are light, electricity, sound, heat, and chemical energy are absorbed or released during chemical reactions. In order to do thermodynamic work, you need to understand the concept of system & surroundings. The system and surroundings must be defined in any example. The system is the area of interest and the surroundings include everything else. Most systems are open and interface with the surroundings. Some systems are closed and do not interface. The total energy in a system and its surroundings do not change. Heat contents of a system is called enthalpy A system is a heat reserve. Changes in energy are denoted by "delta H." Delta H is positive when heat is added and negative when heat is released. Examine the diagrams on page 141. It shows the relationship between a system and its surroundings and how they look on an energy curve. Energy curves are endothermic or exothermic. Enthalpy is stored in chemical bonds and intermolecular forces as potential energy. When substances react, the difference in the enthalpy between reactions and products produces an enthalpy change that can be observed. Standard enthalpy changes are a set of conditions (which should be in your data booklet) are the following: 100 kPa, a concentration of 1 mol/dm3 (1M) for all solutions, and all substances in their standard states. Temperature is usually not a part of these conditions, but is normally included as 298K. Most chemical reactions are exothermic; they give off heat and result in a transfer of energy from system to surroundings. Delta H is negative in exothermic reactions. Endothermic reactions absorb heat and result in a transfer of energy from surroundings to system. Delta H is positive in endothermic reactions. Mission 2: Let's Be Specific About This. Mission Objectives. You should be able to: 1. Describe enthalpy changes and how they are expressed under standard conditions. 2. Calculate specific heat and enthalpy problems. 3. Write and interpret thermochemical equations. A thermochemical equation is a balanced chemical equation that includes the enthalpy change (delta H). Remember that delta H is negative for exothermic reactions and positive for endothermic reactions. Specific heat is the property of a substance which gives heat needed to increase the temperature by 1K. It depends on the number of particles present. The formula for specific heat is Practice Problems: One Two Enthalpy Changes. The direction of change is in the direction of lower stored energy. Chemicals change in a way that reduces their enthalpy. It is expected that a reaction will occur if it leads to a reduction in enthalpy. Products in an exothermic reaction are more stable than the reactants, but stability is a relative term. The sign of delta H is a guide for the likely direction of change, but it is not 100% reliable. Endothermic reactions do occur when there is an increase in the disorder of a system; for example, when gases are produced. Practice problems for Missions 1 & 2. Mission 3: Hess' Law Mission Objectives. You should be able to... 1. Complete enthalpy calculations using Hess' Law. Hess' Law is an application of the conservation of energy law. A chemical equation shows the net reaction; it is a summary of a number of different reactions which are added together and result in an overall reaction. We will spend time working on questions in class. Mission 4: Bond Enthalpy. Mission Objectives. You should be able to... 1. Calculate the enthalpy changes from known bond enthalpy and comparison of these with experimentally measured values. 2. Sketch and evaluate potential energy profiles in determining whether reactants or products are more stable and if the reaction is endothermic or exothermic. 3. Discuss the bond strength in ozone relative to oxygen and its importance to the atmosphere. Mr. Thornley is on the case. You will want to take notes from this video. He lays it out all clear and proper-like. Below are two different energy profiles. The video that follows goes into detail about each profile. You need to make sure you are able to read and interpret each profile. HOMEWORK: Practice problems from the text and the packet.
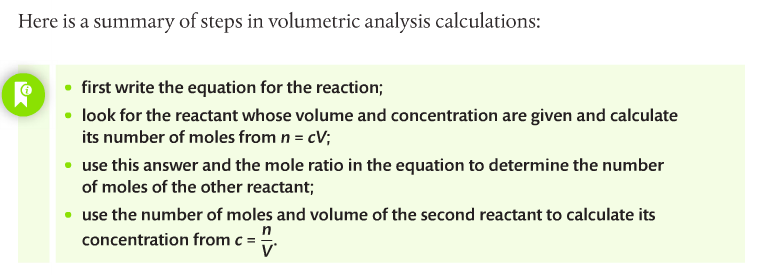
Housekeeping: We will finish up Chapter 1 this week. Your exam over Ch 1 is scheduled for November 24th. Your LR lab (final) is due November 30th. Other than that, unless y'all know something I don't, our house is clean. Agenda: 1. Solution Stoichiometry 2. Gas Laws Content Review: Textbook Readings: Section 1.3 Student Missions: Mission 1: The Problem is the Solution! Mission Objectives. You should be able to... 1. Describe the components of a solution 2. Calculate concentration of a solution using the c = n/V equation and c1V1=c2V2 equation. Section 1.3: Solutions gives you an overview of solution chemistry. Terms to know: solute, solvent, concentration, dilute, stock solution, volumetric analysis and titration. Molar concentration of a solution is defined as the amount in moles of a substance dissolved in 1L (1 dm-3) of a solvent (usually water). The formula for concentration is on page 31 of your textbook. Doc Brown's chemistry page has a nice summary regarding concentration. Remember, however, that the term "molarity" is falling out of use, and instead "c" for concentration should be used in its place. You should go through the practice problems which include the solutions (get it?). You have to complete a titration practical. Quantitative analysis includes a range of techniques to determine the amount or concentration of an analyte. An analyte is the substance under analysis. Volumetric analysis is a quantitative technique used by chemists involving two solutions. A titration involves a standard solution of known concentration which is added to a solution of unknown concentration until the reaction is complete. The reaction progress is monitored through color changes using indicators. 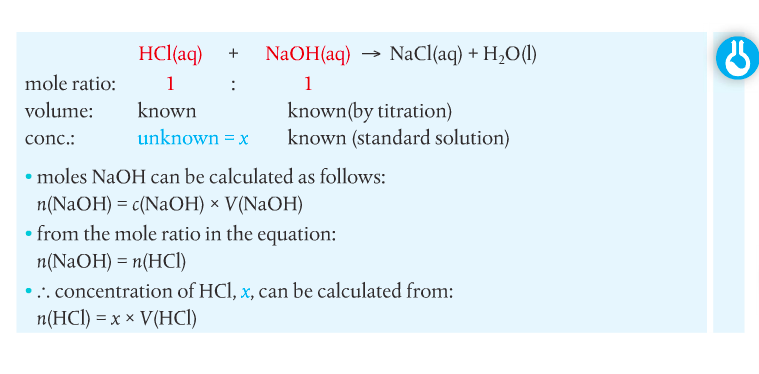 Homework. Solutions are included so you can check your work. Concentration Dilution Solution Preparation Mission 2: The Gas Laws Mission Objectives. You should be able to... 1. Explain the Kinetic Theory of Gases. 2. Describe conditions at STP. 3. Complete calculations using gas laws. You can check your work below.
|
|||||||||||||
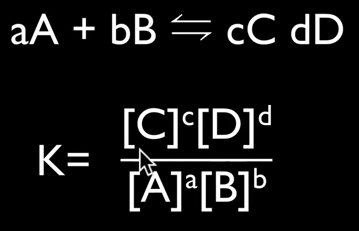


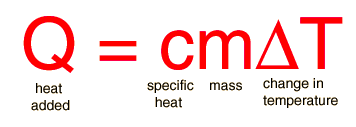
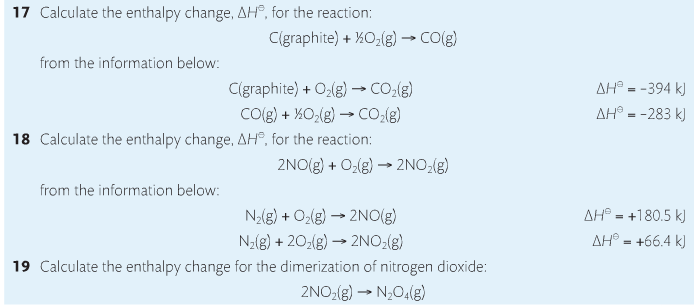
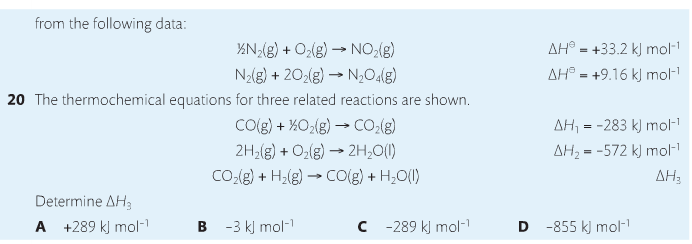

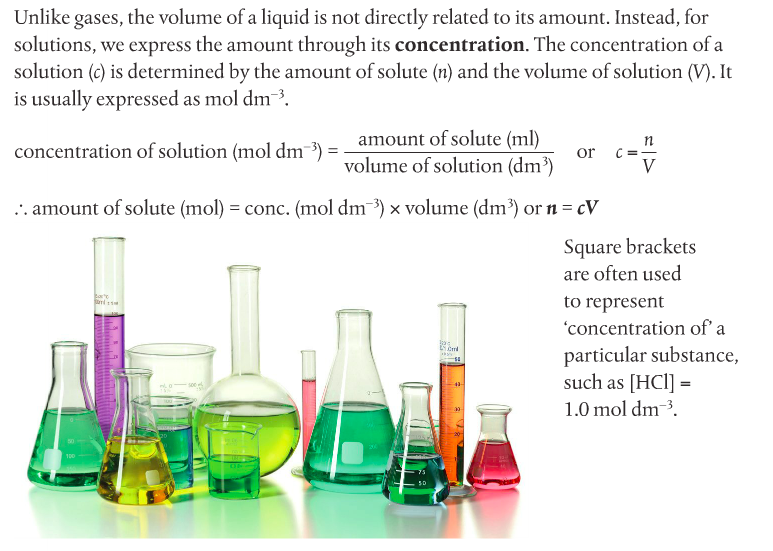


 RSS Feed
RSS Feed