Housekeeping: We are moving on to the second chunk of Chapter 2: proteins, enzymes and nucleic acids. Our schedule does not allow for me to give up five days. Just keep your notes in order and you'll be fine. We will review for the quiz on October 6.
Content Review:
Chapter 2 PowerPoint
Macromolecules Functional Groups
Mad!Science!Links: 2.4, 2.5, & 2.6
Textbook Readings: Chapter 2, sections 2.4, 2.5, 2.6
Workbook Lessons:
Mission 1: Enzymes! Catalyzing Reactions Since...Forever!!!
Misson Objectives. You should be able to...
1. Sketch and describe how enzymes and substrates bind together.
2. Graphically represent the effects of temperature, pH and concentration on enzyme activity.
3. Define "denaturation" and describe the process as it relates to enzymes.
4. Explain how enzymes are used in industry.
Make sure you pay attention to the effects of temperature, pH and concentration on enzyme activity. Try to sketch a graph of what each effect would look like.
Take a look at this animation. Then let's watch the videos.
Content Review:
Chapter 2 PowerPoint
Macromolecules Functional Groups
Mad!Science!Links: 2.4, 2.5, & 2.6
Textbook Readings: Chapter 2, sections 2.4, 2.5, 2.6
Workbook Lessons:
Mission 1: Enzymes! Catalyzing Reactions Since...Forever!!!
Misson Objectives. You should be able to...
1. Sketch and describe how enzymes and substrates bind together.
2. Graphically represent the effects of temperature, pH and concentration on enzyme activity.
3. Define "denaturation" and describe the process as it relates to enzymes.
4. Explain how enzymes are used in industry.
Make sure you pay attention to the effects of temperature, pH and concentration on enzyme activity. Try to sketch a graph of what each effect would look like.
Take a look at this animation. Then let's watch the videos.
Task #1. Get to know enzymes on an interactive level with Bioman's Enzymatic Game. Complete the interactive and take the quiz. You only get one chance. Screen-shot your quiz score and send it to me at kiyra.holt@bisedu.or.id. This score will go in the gradebook.
Mission 2. The Building Blocks of Building Blocks
Misson Objectives. You should be able to...
1. Describe the structure of DNA and RNA.
2. Compare and contrast the structures of DNA and RNA.
3. Sketch and annotate both structures and explain what 5' and 3' mean.
4. Define and distinguish betweens purines and pyrimidines.
Just like monosaccharides are the building blocks for carbohydrates and amino acids are the building blocks for proteins, nucleotides are building blocks for nucleic acids. There are three primary examples of nucleic acids: ATP, DNA and RNA. They are polymers (long chains) of nucleotides.
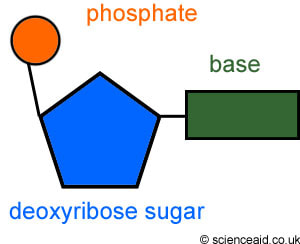
A nucleotide has three parts: a phosphate group, a nitrogenous base, and a 5-carbon sugar at its center. The bonds holding a nucleotide together are covalent.
The following diagram is a standard general representation of a nucleotide. Phosphate groups are circles, the sugars are pentagons, and the nitrogenous bases are rectangles. Let's go here to learn about nitrogenous bases. You'll need to distinguish between purines and pyrimidines. Then we'll go here and show some love to my alma mater and former physics professor, Dr. Rod Nave.
Mission 2. The Building Blocks of Building Blocks
Misson Objectives. You should be able to...
1. Describe the structure of DNA and RNA.
2. Compare and contrast the structures of DNA and RNA.
3. Sketch and annotate both structures and explain what 5' and 3' mean.
4. Define and distinguish betweens purines and pyrimidines.
Just like monosaccharides are the building blocks for carbohydrates and amino acids are the building blocks for proteins, nucleotides are building blocks for nucleic acids. There are three primary examples of nucleic acids: ATP, DNA and RNA. They are polymers (long chains) of nucleotides.
A nucleotide has three parts: a phosphate group, a nitrogenous base, and a 5-carbon sugar at its center. The bonds holding a nucleotide together are covalent.
The following diagram is a standard general representation of a nucleotide. Phosphate groups are circles, the sugars are pentagons, and the nitrogenous bases are rectangles. Let's go here to learn about nitrogenous bases. You'll need to distinguish between purines and pyrimidines. Then we'll go here and show some love to my alma mater and former physics professor, Dr. Rod Nave.
The phosphate groups are the same in both DNA and RNA, but the sugars are different (DNA has deoxyribose and RNA has ribose). The nitrogenous bases are slightly different: uracil is found only in RNA and thymine is only in DNA. The other bases (adenine, guanine, and cytosine) are found in both nucleic acids. Condensation reactions allow the nucleotides to bond to one another and form a chain.
RNA is single stranded and DNA is double stranded.
RNA is single stranded and DNA is double stranded.
Homework: Workbook Lessons #54 -56.
 RSS Feed
RSS Feed