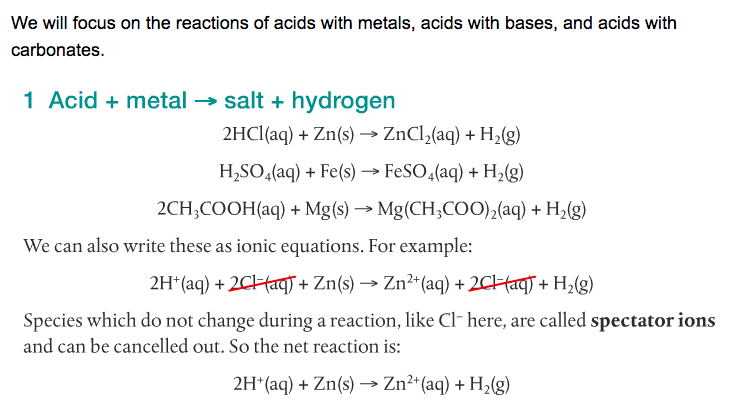Chapter 8: Acids & Bases
Mission 1: Acid-Base Theories.
Mission Objectives. You should be able to:
1. Define, compare and contrast acid/base theories.
2. Explain why Arrhenius' model had to be replaced.
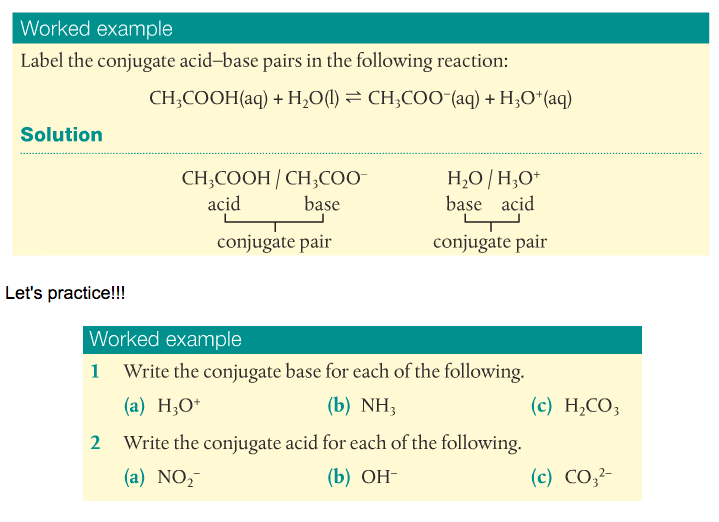
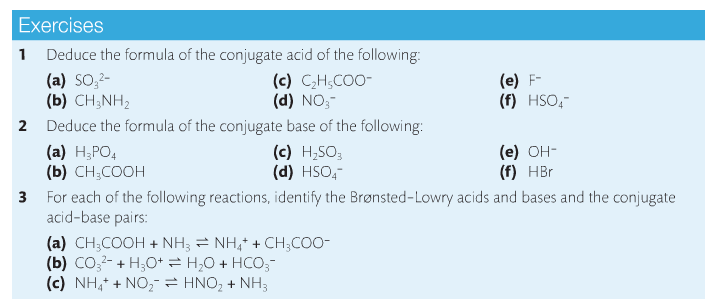
3. Identify conjugate acid/base pairs.
4. Compare and contrast properties of acids & bases.
We will begin with the homie Richard Thornley.
Mission Objectives. You should be able to:
1. Define, compare and contrast acid/base theories.
2. Explain why Arrhenius' model had to be replaced.
3. Identify conjugate acid/base pairs.
4. Compare and contrast properties of acids & bases.
We will begin with the homie Richard Thornley.
There are three known acid-base theories: Arrhenius, Bronsted-Lowry, and Lewis. Arrhenius's theory is very simple: Acids disassociate in water to form H+ ions and bases disassociate to form OH- ions. Bronsted-Lowry (BL)'s definition is more inclusive. Acids are proton donors and bases are proton acceptors. Lewis' definition focuses on the behavior of electrons. Acids accept electron pairs and bases donate electron pairs.
We will focus mainly on Bronsted-Lowry's theory. This leads us to the formation of conjugate acids and bases.
We will focus mainly on Bronsted-Lowry's theory. This leads us to the formation of conjugate acids and bases.
Acids react to form bases and bases react to form acids. The acid-base pairs related to each other like this are called conjugate acid-base pairs and differ by a proton. Substances that can act as an acid and a base are called amphoteric. Water is a good example, as is the HCO3- ion.
A good example is when water becomes the hydronium ion by accepting a proton.
H2O(l) + H+(aq) --> H3O+(aq)
This makes H2O/H3O+ a conjugate acid-base pair.
A good example is when water becomes the hydronium ion by accepting a proton.
H2O(l) + H+(aq) --> H3O+(aq)
This makes H2O/H3O+ a conjugate acid-base pair.
Mission 2: Equal yet Opposite.
Mission Objectives. You should be able to:
1. List and describe the properties of acids and bases.
2. Identify the acids and bases needed to make different salts.
3. Explain the necessity of indicators.
Acids and bases have different characteristics. Acids taste sour and are sometimes sticky. They are corrosive. Acids turn blue litmus red, yellow methyl orange into red, and are colorless in the presence of phenolphthalein. Bases taste bitter and are usually slippery. They are caustic. Bases turn red litmus blue and are pink in the presence of phenolphthalein. At the bottom of this blog post is a T-chart with more acid-base properties.
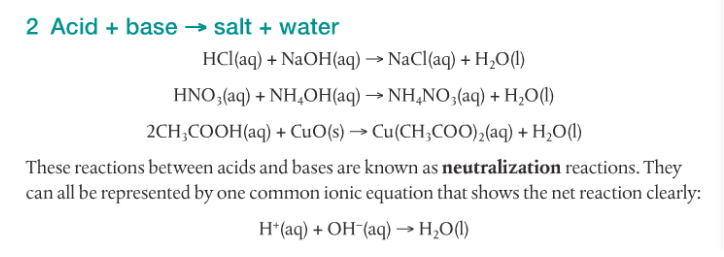
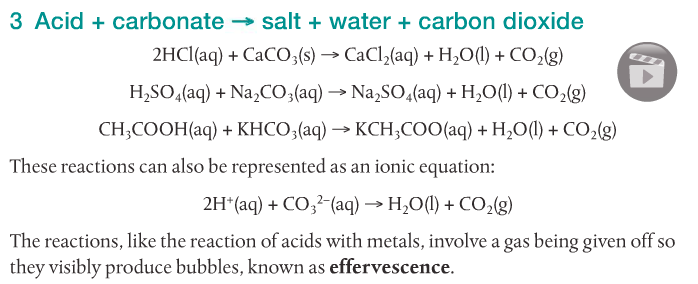
Acids react with bases, metals and carbonates to form salts. The following video shows five different reactions of acids and their products.
Mission Objectives. You should be able to:
1. List and describe the properties of acids and bases.
2. Identify the acids and bases needed to make different salts.
3. Explain the necessity of indicators.
Acids and bases have different characteristics. Acids taste sour and are sometimes sticky. They are corrosive. Acids turn blue litmus red, yellow methyl orange into red, and are colorless in the presence of phenolphthalein. Bases taste bitter and are usually slippery. They are caustic. Bases turn red litmus blue and are pink in the presence of phenolphthalein. At the bottom of this blog post is a T-chart with more acid-base properties.
Acids react with bases, metals and carbonates to form salts. The following video shows five different reactions of acids and their products.
It is my plan to have you guys doing your own variations of these demonstrations later in the week. Whenever possible, it's always better to do it yourself. You may get different results and will have to explain why. Don't be afraid.
Indicators are chemical detectors. Acids and bases change color depending on which indicator is used. The most common indicator is litmus (turns red in the presence of acid and blue in the presence of base). Phenolphthalein turns pink in the presence of a base, but is colorless when combined with an acid. Universal indicator is a combination of many other indicators that turns a variety of colors depending on the concentration of H+ in solution.
Image courtesy of https://tackk.com/qbel4z
Indicators are chemical detectors. Acids and bases change color depending on which indicator is used. The most common indicator is litmus (turns red in the presence of acid and blue in the presence of base). Phenolphthalein turns pink in the presence of a base, but is colorless when combined with an acid. Universal indicator is a combination of many other indicators that turns a variety of colors depending on the concentration of H+ in solution.
Image courtesy of https://tackk.com/qbel4z
Let's practice!!!
Below are some characteristics of acids & bases.
|
Acids
Hydrogen ion donor Sour Can burn Conductive Reacts with metals to produce hydrogen gas Reacts with carbonates to produce carbon dioxide gas Turns blue litmus red Red to orange range for universal indicator pH < 7 More [H+] |
Bases
Hydrogen ion acceptor Bitter Slippery Can burn Conductive Dissolve fats & oils Turns red litmus blue Blue to purple range for universal indicator pH > 7 More [OH-] |
Mission 3: All About That pH!
Mission Objectives. You should be able to:
1. Explain the arrangement of the pH scale in terms of acidity, neutrality and alkalinity.
2. Understand that a change of one on a pH scale represents a change of 10X in the [H+].
3. Calculate pH/pKa and pOH/pKb given a formula.
4. Write and explain the ion product constant and expression for water.
We will start this lesson with an overview. Mr. Thornley tagged Mr. Anderson and so now he will jump into the ring and do his thing. We will work on some practice problems in class and for homework. It's important that you be able to calculate pH and pOH, as well as pKa and pKb. HL, you will also need to be able to calculate Ka from pKa and Kb from pKb. Learn how.
Mission Objectives. You should be able to:
1. Explain the arrangement of the pH scale in terms of acidity, neutrality and alkalinity.
2. Understand that a change of one on a pH scale represents a change of 10X in the [H+].
3. Calculate pH/pKa and pOH/pKb given a formula.
4. Write and explain the ion product constant and expression for water.
We will start this lesson with an overview. Mr. Thornley tagged Mr. Anderson and so now he will jump into the ring and do his thing. We will work on some practice problems in class and for homework. It's important that you be able to calculate pH and pOH, as well as pKa and pKb. HL, you will also need to be able to calculate Ka from pKa and Kb from pKb. Learn how.
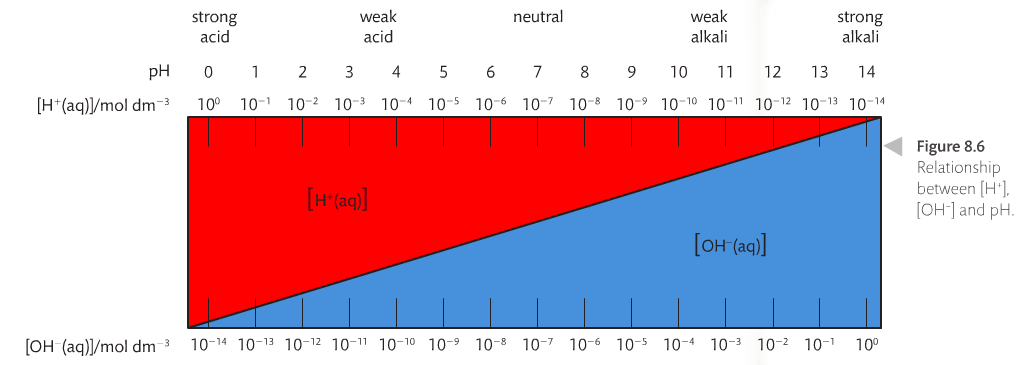
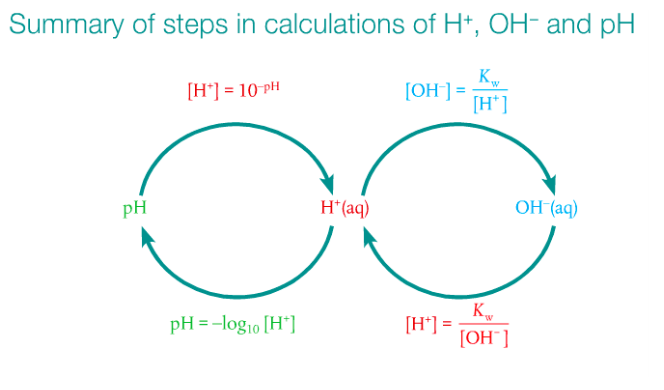
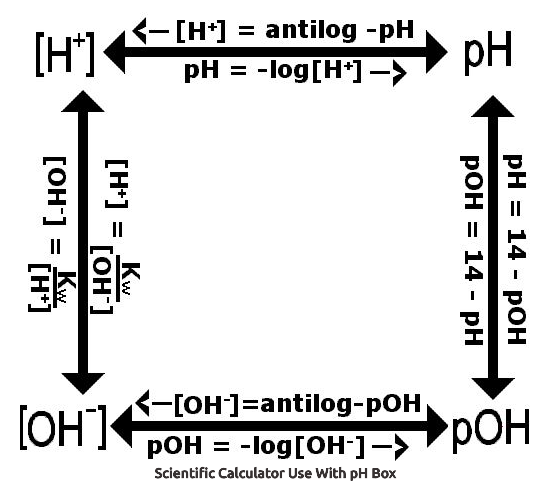
pH stands for hydrogen power. To calculate pH, you need the equation pH = -log [H+]. If you work in reverse, then the formula becomes [H+] is base 10 raised to the power of -pH. But it is essential you understand the context for what pH numbers mean. A solution that has [H+] = 0.1 mol dm-3 has a pH of 1. A solution that has [H+] = 0.01 mol dm-3 has a pH of 2, and so on and so forth. The relationship between pH and [H+] is inverse; the higher the pH, the lower the [H+]. A change in 1 on a pH scale represents a change by 10-fold for the [H+].
What is the ion product constant of water and why does it matter? What do we use it for?
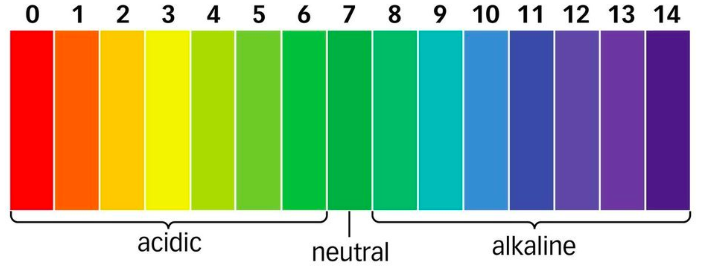
pH values go from 1 to 14, with 1-6 being acidic (more [H+]) and 8-14 being alkaline (less [H+]). pH numbers are positive and have no units.
pOH stands for hydroxide power. This is the opposite of pH. Adding pH to pOH should equal 14. The relationship between pH and pOH is illustrated below.
Images courtesy of the Pearson text.
What is the ion product constant of water and why does it matter? What do we use it for?
pH values go from 1 to 14, with 1-6 being acidic (more [H+]) and 8-14 being alkaline (less [H+]). pH numbers are positive and have no units.
pOH stands for hydroxide power. This is the opposite of pH. Adding pH to pOH should equal 14. The relationship between pH and pOH is illustrated below.
Images courtesy of the Pearson text.
Below image courtesy of Wyzant.com.
Mission 4: Acid-Base Titration
Mission Objectives. You should be able to:
1. Understand the nature of titration.
2. Titrate a known acid with an unknown base.
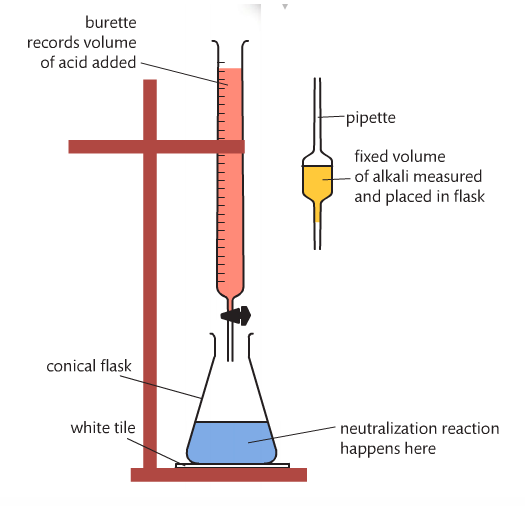
A titration is a volumetric analysis technique that involves a reaction between a solution of unknown concentration (c) and a standardized solution (titrant). The titrant is in the buret and the unknown concentration (analyte) is in the flask. The objective is to determine the unknown concentration by reaching the equivalence point (when the acid neutralizes the base).
Image from the Pearson text.
Mission Objectives. You should be able to:
1. Understand the nature of titration.
2. Titrate a known acid with an unknown base.
A titration is a volumetric analysis technique that involves a reaction between a solution of unknown concentration (c) and a standardized solution (titrant). The titrant is in the buret and the unknown concentration (analyte) is in the flask. The objective is to determine the unknown concentration by reaching the equivalence point (when the acid neutralizes the base).
Image from the Pearson text.
Here is a writeup of a titration lab.
The first video goes through the setup of and actual titration. The second video shows how to do the math that goes along with it.
The first video goes through the setup of and actual titration. The second video shows how to do the math that goes along with it.
pH curves or titration curves. A titration curve is the plot of the pH of the analyte solution versus the volume of the titrant added as the titration progresses (Khan Academy, 2018). You need to be familiar with the four basic pH curves and the roles of the different indicators used in titration. I strongly recommend watching the below videos. For the first video, start at 3:00.
Mission 5: What Are You, Strong or Weak?
Mission Objectives. You should be able to...
1. Differentiate between strong and weak acids and bases.
2. List the properties of strong and weak acids and bases.
Strong acids and bases disassociate completely in water. This means that the acid/base breaks up completely into their component ions. By contrast, weak acids and bases disassociate only partially. If you look at the ionization of a strong acid/base, you will see that it is a forward reaction only. Examining the ionization of a weak acid/base requires an equilibrium reaction.
Properties of strong acids & bases:
1. Strong acids/bases conduct electricity very well due to the number of ions in solution. 2. Reaction rates using strong acids/bases increase due to the number of ions in solution.
3. For strong acids, the higher the [H+], the lower the pH. For strong bases, the lower the [H+], the higher the pH.
4. Strong acids are good proton donors and have weak conjugate bases.
5. Strong bases are good proton acceptors and have weak conjugate acids.
Strong acids: hydrochloric acid, nitric acid, sulfuric acid. All others are WEAK.
Strong bases: sodium hydroxide, potassium hydroxide, rubidium hydroxide and barium hydroxide. All others are WEAK.
Mission Objectives. You should be able to...
1. Differentiate between strong and weak acids and bases.
2. List the properties of strong and weak acids and bases.
Strong acids and bases disassociate completely in water. This means that the acid/base breaks up completely into their component ions. By contrast, weak acids and bases disassociate only partially. If you look at the ionization of a strong acid/base, you will see that it is a forward reaction only. Examining the ionization of a weak acid/base requires an equilibrium reaction.
Properties of strong acids & bases:
1. Strong acids/bases conduct electricity very well due to the number of ions in solution. 2. Reaction rates using strong acids/bases increase due to the number of ions in solution.
3. For strong acids, the higher the [H+], the lower the pH. For strong bases, the lower the [H+], the higher the pH.
4. Strong acids are good proton donors and have weak conjugate bases.
5. Strong bases are good proton acceptors and have weak conjugate acids.
Strong acids: hydrochloric acid, nitric acid, sulfuric acid. All others are WEAK.
Strong bases: sodium hydroxide, potassium hydroxide, rubidium hydroxide and barium hydroxide. All others are WEAK.
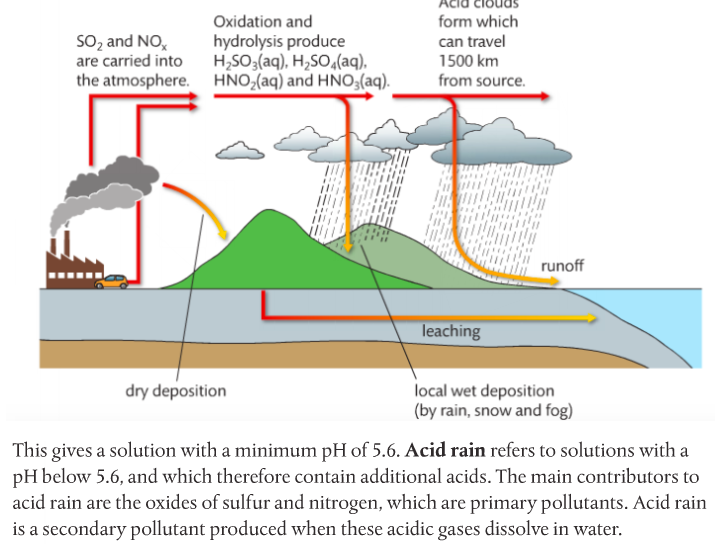
Mission 6: Acid Deposition.
Mission Objectives. You should be able to...
1. List the compounds that make up acid rain.
2. Describe the sources of the oxides of sulfur and nitrogen.
3. State the effects of acid deposition.
Mission Objectives. You should be able to...
1. List the compounds that make up acid rain.
2. Describe the sources of the oxides of sulfur and nitrogen.
3. State the effects of acid deposition.