Molecular Biology: Sections 2.1, 2.2 & 2.3
Essential Ideas:
2.1: Living organisms control their composition by a complex web of chemical reactions.
2.2: Water is the medium of life
2.3: Compounds of carbon, hydrogen and oxygen are used to supply and store energy.
2.1: Living organisms control their composition by a complex web of chemical reactions.
2.2: Water is the medium of life
2.3: Compounds of carbon, hydrogen and oxygen are used to supply and store energy.
Mission 1: Molecules to Metabolism
Mission Objectives: You should be able to...
1. Describe the significance of the carbon atom in terms of the structures that it can form.
2. Describe the significance of carbon in terms of life.
3. Explain the process of metabolism.
4. Compare and contrast anabolism and catabolism.
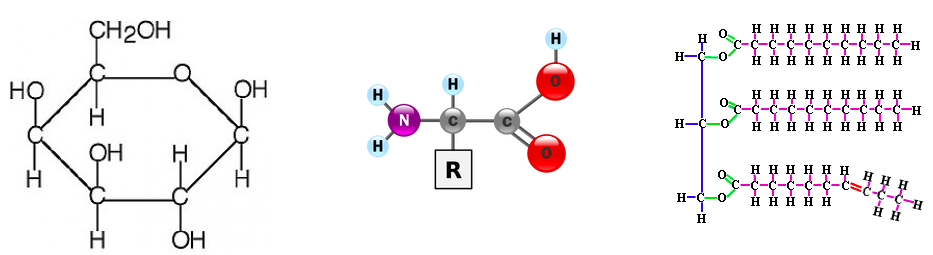
5. Draw molecular diagrams of glucose, ribose, a general amino acid and a saturated fatty acid.
6. Identify biochemicals such as sugars, lipids, and amino acids from diagrams.
Mission Objectives: You should be able to...
1. Describe the significance of the carbon atom in terms of the structures that it can form.
2. Describe the significance of carbon in terms of life.
3. Explain the process of metabolism.
4. Compare and contrast anabolism and catabolism.
5. Draw molecular diagrams of glucose, ribose, a general amino acid and a saturated fatty acid.
6. Identify biochemicals such as sugars, lipids, and amino acids from diagrams.
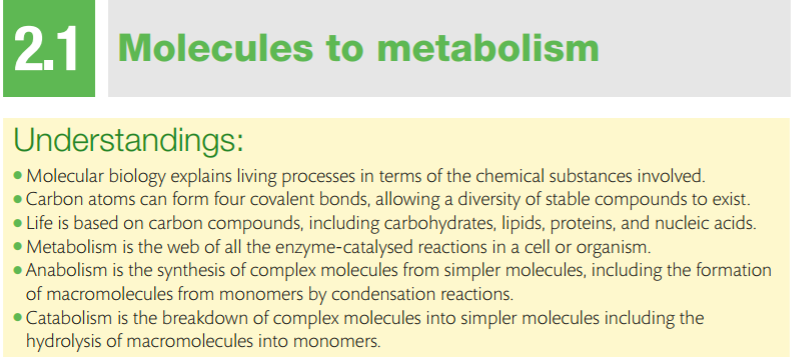
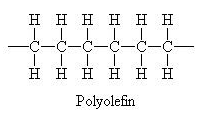
Carbon atoms can form four covalent bonds, which allows a diversity of stable compounds to exist. As a result, carbon-containing organic molecules can form chains, rings, and branched chains.
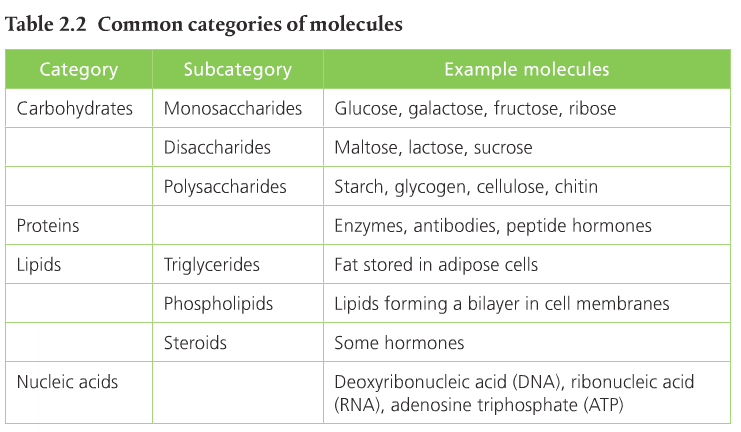
These different structures allow for the creation of macromolecules. Macromolecules are, quite obviously, large molecules. There are four kinds: carbohydrates, proteins, lipids, and nucleic acids. Each macromolecule has its own building blocks. Carbohydrates are composed of monosaccharides (simple sugars), proteins are composed of amino acids, lipids are composed of glycerol, fatty acids, and phosphate groups, and nucleic acids are formed from nucleotides.
Metabolism
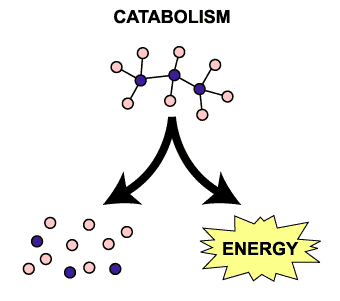
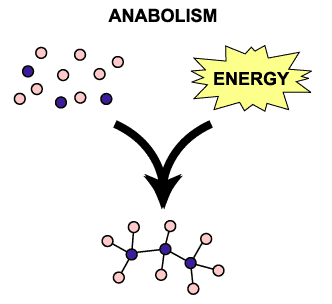
The ways in which macromolecules interact with each other in living organisms. These interactions are collectively called metabolism. Metabolism has two components: catabolism and anabolism.
The ways in which macromolecules interact with each other in living organisms. These interactions are collectively called metabolism. Metabolism has two components: catabolism and anabolism.
|
Catabolism
Reactions that break down complex molecules (food) into smaller, simpler molecular forms. The food is digested (hydrolyzed) by enzymes. Each reaction is called a hydrolysis and requires a molecule of water as a reactant. On page 57 (in the HL text), there is an image of lactose (a disaccharide) being hydrolyzed into glucose and galactose (two monosaccharides). The way to recognize a hydrolysis reaction is to look at where the water molecule is; as a reactant, it should always be on the LEFT side of the arrows. |
Anabolism
Reactions that convert simple molecules into larger, more complex molecules. They occur to re-form the larger, biochemically important molecules. If you reverse the reactions on page 57, each one becomes a condensation reaction. These reactions produce water as a product. Water will appear on the RIGHT side of the arrow. Condensation reactions require different enzymes than hydrolysis reactions; ones that create covalent bonds as opposed to breaking them. |
Mission 2: Water
Mission Objectives: You should be able to...
1. Sketch and describe a water molecule.
2. Explain what it means to be "polar."
3. List and describe and explain the different properties of water.
4. Be able to compare and contrast water and methane.
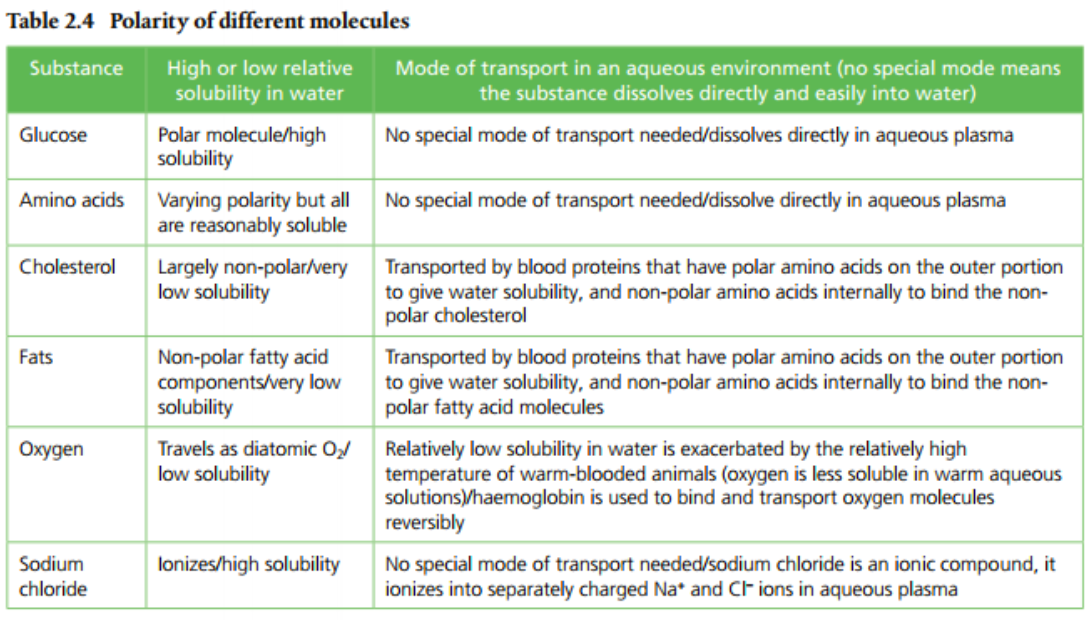
5. Summarize the the modes of transport of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in blood in relation to their solubility in water.
Mission Objectives: You should be able to...
1. Sketch and describe a water molecule.
2. Explain what it means to be "polar."
3. List and describe and explain the different properties of water.
4. Be able to compare and contrast water and methane.
5. Summarize the the modes of transport of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in blood in relation to their solubility in water.
|
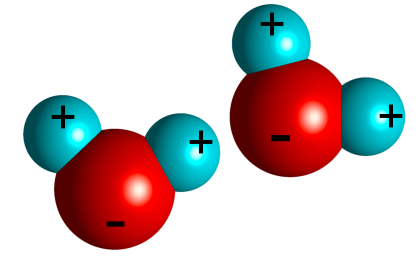
Water is the medium of life. Understanding why it is so important requires understanding of its structure. There are two hydrogen ions sharing electrons with an oxygen ion. Sharing of electrons results in a covalent bond. The water molecule is polar, meaning that it has a net charge on one end (the oxygen end). As a result, water can interact with itself and other molecules in different ways. Water has many different properties which makes it so important in living organisms.
|
Cohesion & Adhesion
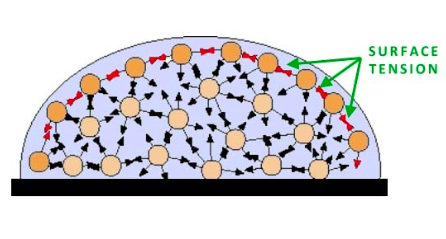
Cohesion is when molecules of the same type are attracted to each other. When the positive end of one water molecule attracts the negative end of another, a hydrogen bond occurs. This allows for water to form droplets, have a surface tension, and move as a "column" in plant vascular tissue. Surface tension is the strong cohesive forces of water molecules at the surface, which forms a layer or film that makes it difficult to move an object over the surface. Adhesion is the attraction of unlike molecules, such as water and cellulose. When water moves up through a capillary tube, both cohesion and adhesion are at work. Cohesion pulls the water up the tube; adhesion keeps the water from falling down. |
Thermal Properties
Water has thermal properties. It has a very high specific heat, which is the ability to absorb or give off a great deal of heat without changing its internal temperature. Water also has a high heat of vaporization, which means it absorbs a great deal of heat when it evaporates. We use this property as a cooling mechanism, when we sweat.
Solvent Properties
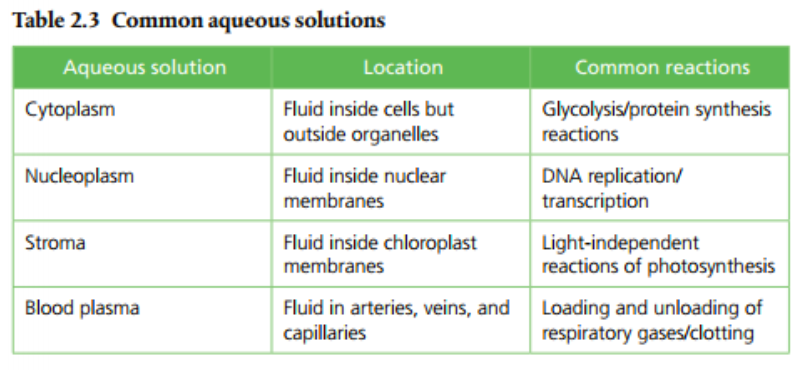
Water can dissolve a lot of substances due to its polarity. The vast majority of molecules found in and out of cells are also polar molecules. This means they can be dissolved in water. Lipids, however, are non-polar and require special strategies to deal with their biochemistry and transport. Aqueous solutions are substances dissolved in water. Page 64 (Table 2.3) provides a list of some common aqueous solutions.
Because of these properties, water is an excellent transport medium. Plants have vascular tissue that carries aqueous solutions from the roots to the leaves and vice versa. In animals, blood is the main transport medium and is primarily made up of water. The liquid portion of blood is called blood plasma, and it can contain glucose, amino acids, proteins and certain ions that can transport carbon dioxide.
Substances can be hydrophobic or hydrophilic. Hydrophobic means "water hating" and hydrophilic means "water loving." Most of the molecules are obviously hydrophilic, otherwise they wouldn't be soluble in water. Hydrophobic molecules include lipids and hydrocarbons, which are non-polar and aren't soluble in water.
Proteins can go both ways based on the location of the amino acid within the three-dimensional structure of the protein.
Source: Pearson (2014)
Water has thermal properties. It has a very high specific heat, which is the ability to absorb or give off a great deal of heat without changing its internal temperature. Water also has a high heat of vaporization, which means it absorbs a great deal of heat when it evaporates. We use this property as a cooling mechanism, when we sweat.
Solvent Properties
Water can dissolve a lot of substances due to its polarity. The vast majority of molecules found in and out of cells are also polar molecules. This means they can be dissolved in water. Lipids, however, are non-polar and require special strategies to deal with their biochemistry and transport. Aqueous solutions are substances dissolved in water. Page 64 (Table 2.3) provides a list of some common aqueous solutions.
Because of these properties, water is an excellent transport medium. Plants have vascular tissue that carries aqueous solutions from the roots to the leaves and vice versa. In animals, blood is the main transport medium and is primarily made up of water. The liquid portion of blood is called blood plasma, and it can contain glucose, amino acids, proteins and certain ions that can transport carbon dioxide.
Substances can be hydrophobic or hydrophilic. Hydrophobic means "water hating" and hydrophilic means "water loving." Most of the molecules are obviously hydrophilic, otherwise they wouldn't be soluble in water. Hydrophobic molecules include lipids and hydrocarbons, which are non-polar and aren't soluble in water.
Proteins can go both ways based on the location of the amino acid within the three-dimensional structure of the protein.
Source: Pearson (2014)
Mission 3: Carbohydrates & Lipids.
Mission Objectives: You should be able to...
1. Explain how monomers become polymers.
2. Differentiate between saturated, monounsaturated and polyunsaturated fatty acids.
3. Explain the difference between cis- and trans-.
4. Describe and explain the structure and function of cellulose and starch in plants and glycogen in humans.
5. Explain why lipids are more suitable for long-term energy storage in humans than carbohydrates.
6. Calculate BMI.
Mission Objectives: You should be able to...
1. Explain how monomers become polymers.
2. Differentiate between saturated, monounsaturated and polyunsaturated fatty acids.
3. Explain the difference between cis- and trans-.
4. Describe and explain the structure and function of cellulose and starch in plants and glycogen in humans.
5. Explain why lipids are more suitable for long-term energy storage in humans than carbohydrates.
6. Calculate BMI.
|
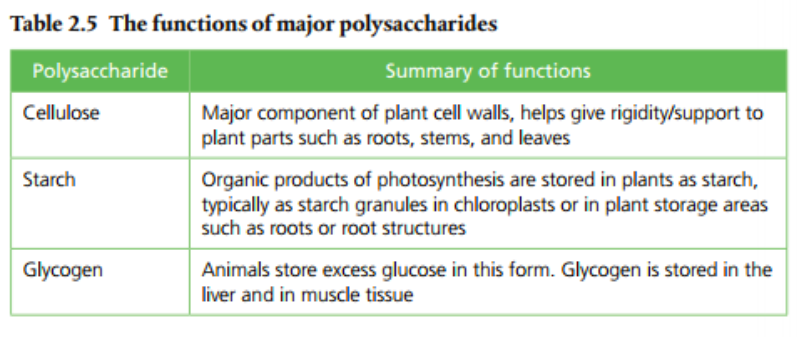
Monosaccharides are simple sugars that combine to make larger molecules. Examples of monosaccharides are glucose, fructose & galactose. Disaccharides are combinations of two monosaccharides. Examples include maltose, lactose, and sucrose. Maltose is a combination of two glucose molecules. Lactose is a combination of glucose and galactose. Sucrose is a combination of glucose and fructose. Polysaccharides are chains of monosaccharides that are used in energy storage. Examples include starch, glycogen, and cellulose. Page 71 in the SL text describes the functions of each.
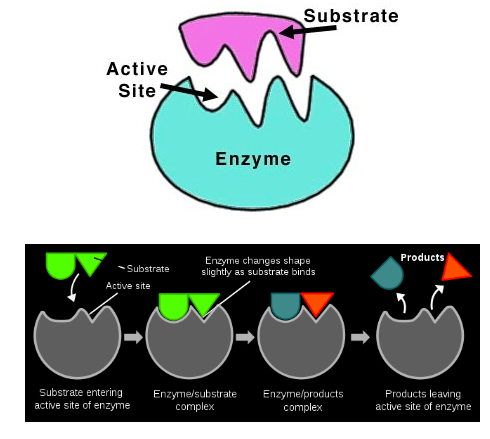
Disaccharides and polysaccharides are constructed in condensation reactions that remove water from the process. For these reactions to take place, the molecules must collide in a particular orientation, at a particular speed, and depends on the identity of the reactant. To increase the chances that biochemical reactions take place, enzymes are used. They have specific shapes in which a reactant can fit, at a molecular location called the active site. Most biochemical reactions are catalyzed by enzymes. Images courtesy of biology.tutorvista.com. |
Examples of biochemical reactions:
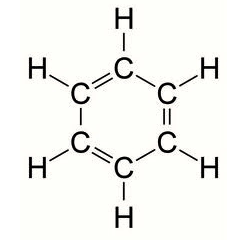
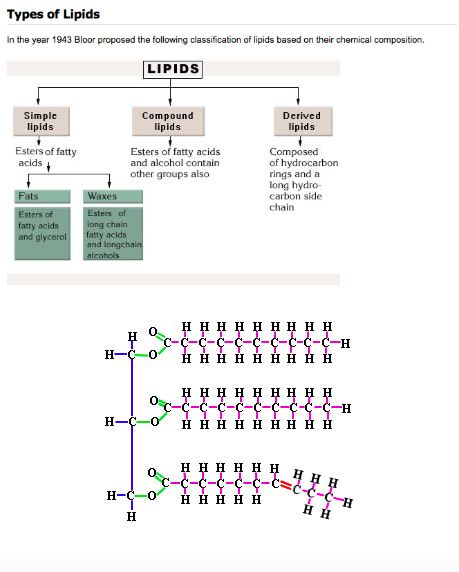
Lipids are molecules that have a variety of functions. They include: long term energy storage (way more efficient that carbohydrates), thermal insulation, and buoyancy. They are composed of a glycerol molecule and a fatty acid tail. You already know of one kind of lipid; the phospholipid bilayer of the plasma membrane. All fatty acids have a carboxyl group (-COOH) at one end and a methyl group (CH3-) at the other end. In between is a hydrocarbon chain usually between 11 and 23 carbon atoms long (not counting the methyl group carbon). Saturated fatty acids occur when all of the carbons are carrying as many hydrogen atoms as possible. This means that they're saturated with hydrogen. Since a saturated fat has no double bonds, the molecule is a straight chain. Unsaturated fatty acids, of course, are the opposite. Not every carbon contains a full set of hydrogen atoms, and there is a double bond between two carbon atoms, which bends the molecule. Naturally curved fatty acids (those with the kink) are called cis fatty acids and hydrogenated fatty acids (those that have had hydrogen atoms added and been straightened as a result) are called trans fatty acids. |
Review PowerPoint
| ib_bio_ppt_1.pptx | |
| File Size: | 4873 kb |
| File Type: | pptx |