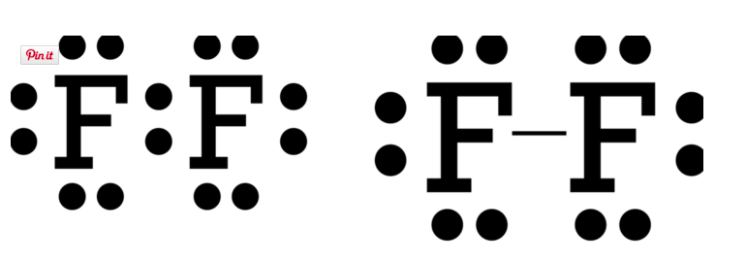Chemical Bonding I: Ionic & Covalent Bonds
Student Missions:
Mission 1: Ions, Ions, Ions!
Mission Objectives. By the end of this lesson, you should be able to:
1. Determine which elements form cations and which elements form anions.
2. Describe the structure of an ionic bond based on valence electrons.
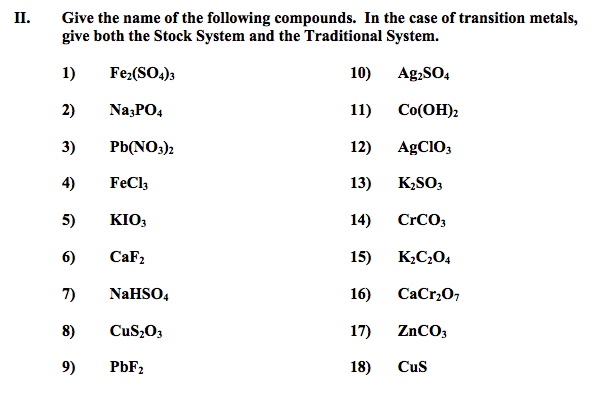
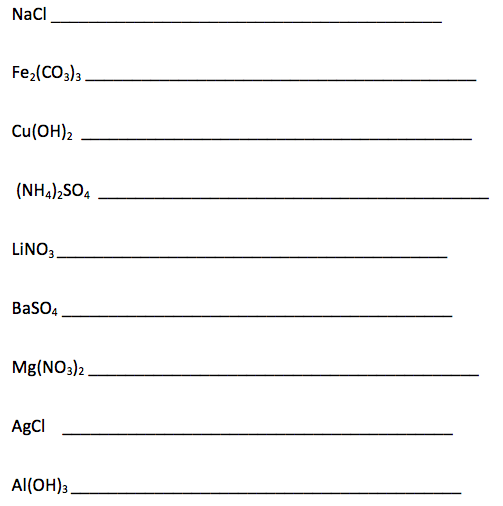
3. Deduce the name and formula of an ionic compound from its component ions, including polyatomic ions.
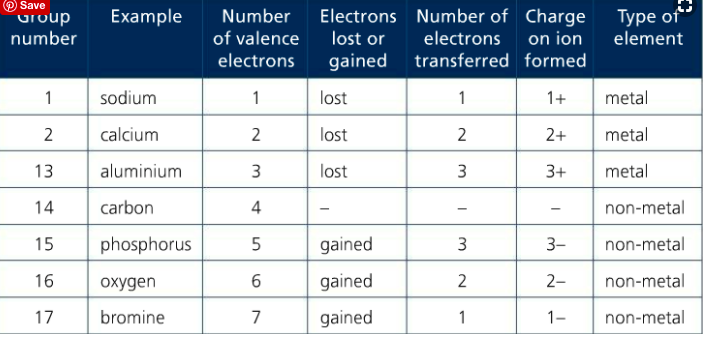
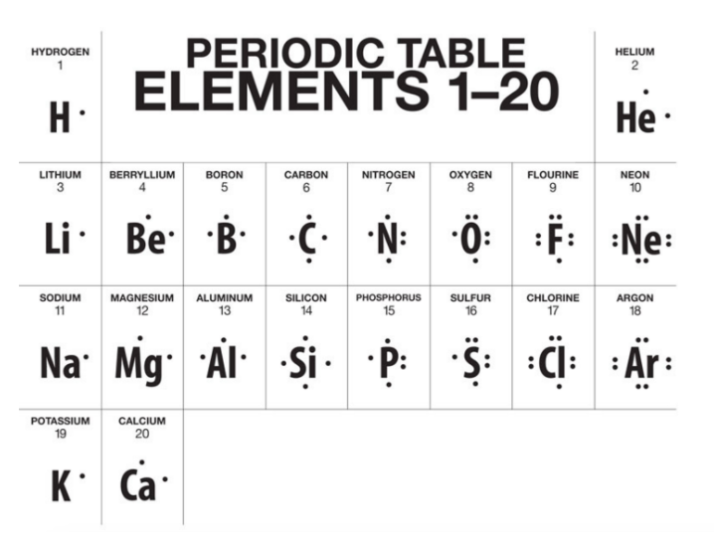
Recall that the group number on the periodic table represents the number of valence electrons in that group. Also recall that group 18, the noble gases, have stable octets. The objective for any element that is not a noble gas is to lose or gain electrons to have a noble gas configuration. Stability is key.
Elements in G1 will LOSE their valence electrons to form ions with a +1 charge. Elements in G2 will LOSE theirs to form ions with a +2 charge. Elements in G13 will lose theirs to form ions with a +3 charge. Any time an element LOSES a valence electron, a cation is formed. Cations are positively charged ions. Metallic elements form cations.
Conversely, elements in G15 will GAIN 3 electrons to form ions with a -3 charge. Elements in G16 will GAIN 2 electrons to form ions with a -2 charge, and elements in G17 will GAIN 1 electron to form an ion with a -1 charge. Any time an element GAINS a valence electron, an anion is formed. Anions are negatively charged ions. Nonmetals form anions.
Mission 1: Ions, Ions, Ions!
Mission Objectives. By the end of this lesson, you should be able to:
1. Determine which elements form cations and which elements form anions.
2. Describe the structure of an ionic bond based on valence electrons.
3. Deduce the name and formula of an ionic compound from its component ions, including polyatomic ions.
Recall that the group number on the periodic table represents the number of valence electrons in that group. Also recall that group 18, the noble gases, have stable octets. The objective for any element that is not a noble gas is to lose or gain electrons to have a noble gas configuration. Stability is key.
Elements in G1 will LOSE their valence electrons to form ions with a +1 charge. Elements in G2 will LOSE theirs to form ions with a +2 charge. Elements in G13 will lose theirs to form ions with a +3 charge. Any time an element LOSES a valence electron, a cation is formed. Cations are positively charged ions. Metallic elements form cations.
Conversely, elements in G15 will GAIN 3 electrons to form ions with a -3 charge. Elements in G16 will GAIN 2 electrons to form ions with a -2 charge, and elements in G17 will GAIN 1 electron to form an ion with a -1 charge. Any time an element GAINS a valence electron, an anion is formed. Anions are negatively charged ions. Nonmetals form anions.
Transition metals have electron configurations that allow them to lose different numbers of electrons from their d subshell and form ions with different oxidation states. An oxidation state is the charge on an ion. The ions have different properties, such as forming compounds with different colors. An example would be the copper I and copper II ions ( Cu+ and Cu+2). One is green, the other blue.
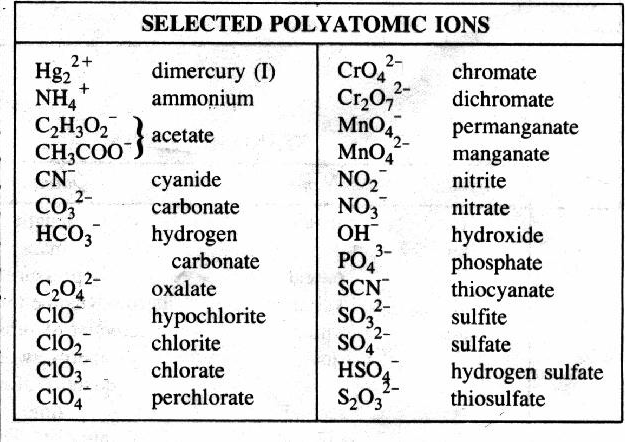
Polyatomic ions are ions that are made up of several atoms covalently bonded, but have a positive or negative charge. I have given you a ion reference chart to use in class, but you need to memorize the different polyatomic ions, as they are not in the data booklet and you cannot use the ion chart on the test.
Image courtesy of tinycards.duolingo.com
Polyatomic ions are ions that are made up of several atoms covalently bonded, but have a positive or negative charge. I have given you a ion reference chart to use in class, but you need to memorize the different polyatomic ions, as they are not in the data booklet and you cannot use the ion chart on the test.
Image courtesy of tinycards.duolingo.com
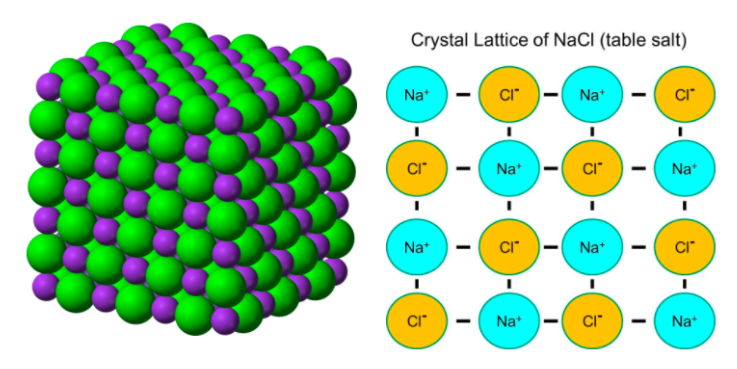
The images above (courtesy of wikipedia.org and http://sphweb.bumc.bu.edu) show the crystal lattice structure of salt (NaCl). The electrostatic attraction between cations and anions causes the ions to surround themselves with the opposite charged ion. The coordinate number is the number of surrounding ions. For salt, the coordination number is 6, as each Na+ is surrounded by 6 Cl-. Because these structures can get big, their formulas are merely the ratios of ions present. The simplest ratio is called a formula unit (fmu).
Lattice energy is a measure of the strength of attraction between ions within the lattice. The energy is greater for small, highly charged ions. Because of their lattice structures, ionic compounds have high melting and boiling points. The electrostatic attraction between the ions are strong and requires a lot of energy to break. Therefore, they are solids at room temperature and melt at very high temperatures.. When the charge is greater, the MPs and BPs are higher.
Volatility is the tendency of a substance to vaporize. Ionic compounds have a low volatility, or is non-existent. This means that they do not tend to vaporize because the bonds are too strong.
Lattice energy is a measure of the strength of attraction between ions within the lattice. The energy is greater for small, highly charged ions. Because of their lattice structures, ionic compounds have high melting and boiling points. The electrostatic attraction between the ions are strong and requires a lot of energy to break. Therefore, they are solids at room temperature and melt at very high temperatures.. When the charge is greater, the MPs and BPs are higher.
Volatility is the tendency of a substance to vaporize. Ionic compounds have a low volatility, or is non-existent. This means that they do not tend to vaporize because the bonds are too strong.
Solubility is the tendency of a substance to dissolve. Partial charges in water (a polar molecule) are attracted to opposite charges in an ionic compound and can dislodge the ions. When ions are surrounded by water, they become hydrated. Then the compound can dissolve. The state symbol for a compound dissolved in water is (aq) for aqueous. Nonpolar solvents do not have a charge, and therefore the ions do not pull apart. As a result, the substance is insoluble.
Conductivity & Brittleness. Based on an ability of a compound to conduct electricity, the ions have to be able to move. Therefore they cannot conduct electricity in the solid (s) state. The compound has to be molten (or liquid (l)) or dissolved in water (aq) to conduct electricity.
The ionic crystal lattice can shatter when a force is applied, so therefore it is brittle.
Homework: Re-read Mission 1. We will be working the below problems in class.
Conductivity & Brittleness. Based on an ability of a compound to conduct electricity, the ions have to be able to move. Therefore they cannot conduct electricity in the solid (s) state. The compound has to be molten (or liquid (l)) or dissolved in water (aq) to conduct electricity.
The ionic crystal lattice can shatter when a force is applied, so therefore it is brittle.
Homework: Re-read Mission 1. We will be working the below problems in class.
Mission 2: Sharing is Caring.
Mission Objectives. You should be able to...
1. Determine when covalent bonds are formed and which elements form them.
2. Predict the name of an ionic compound and a covalent compound.
3. Explain the difference between polar and nonpolar molecules.
Covalent bonds are the result of valence electrons being shared in order to achieve stable octets. If you think back...way back...to the short lesson on Lewis Dot, you'll recall that certain elements contained a certain number of dots that represented their valence electrons.
Mission Objectives. You should be able to...
1. Determine when covalent bonds are formed and which elements form them.
2. Predict the name of an ionic compound and a covalent compound.
3. Explain the difference between polar and nonpolar molecules.
Covalent bonds are the result of valence electrons being shared in order to achieve stable octets. If you think back...way back...to the short lesson on Lewis Dot, you'll recall that certain elements contained a certain number of dots that represented their valence electrons.
Anyhoo, if you take a look at groups 15, 16 & 17 (the nonmetals), this is where covalent bonding takes place. These elements can bond with themselves to share a pair (or two, or three) of electrons to form stable octets. Take a look below. Fluorine will share a pair of electrons with another fluorine and a single covalent bond is formed (represented by a line).
Group 16 elements will share two pairs of electrons (double covalent bond) and group 15 elements will share three pairs of electrons (triple covalent bond). Again, the goal is stability. The Octet Rule rules all.
Here is an interactive on covalent bonding. Play with it.
There are seven elements that are found in nature covalently bonded to each other. They are called diatomic elements. A useful memory aid is HONI Bring Fried Clams. So when you're writing these elements in chemical equations, you need to make sure you write them correctly.
Coordinate covalent bonds are bonds where one atom contributes both bonding electrons. You demonstrate them in structural formulas using an arrow that shows where the electrons came from. Once formed, the coordinate covalent bond is just like any other bond. Carbon monoxide is a good example of this.
Here is an interactive on covalent bonding. Play with it.
There are seven elements that are found in nature covalently bonded to each other. They are called diatomic elements. A useful memory aid is HONI Bring Fried Clams. So when you're writing these elements in chemical equations, you need to make sure you write them correctly.
Coordinate covalent bonds are bonds where one atom contributes both bonding electrons. You demonstrate them in structural formulas using an arrow that shows where the electrons came from. Once formed, the coordinate covalent bond is just like any other bond. Carbon monoxide is a good example of this.
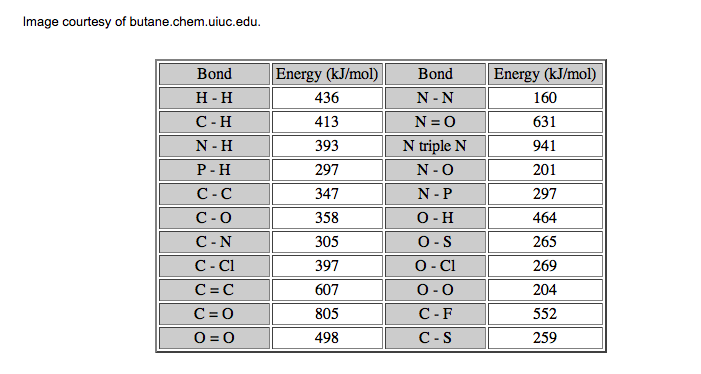
Bond Disassociation Energy. This is the energy required to break covalent bonds. The unit is usually given in kJ/mol, which is the energy required to break one mole of bonds. Look below. Simply put, the larger the BDE, the stronger the covalent bond. Single bonds are easier to break than double bonds, and double bonds are easier to break than triple bonds, and so on and so forth. This will become important when we get to thermochemistry.
Mission 3: Electron Tug-of-War.
Mission Objectives. You should be able to...
1. Explain the difference between polar and nonpolar molecules.
Because covalent bonding involves sharing of electrons, there can be differences in how the electrons are shared between the bonded atoms. The character (or behavior) of the molecules depends on the kind of and number of atoms joined together, which in turn, determine molecular properties.
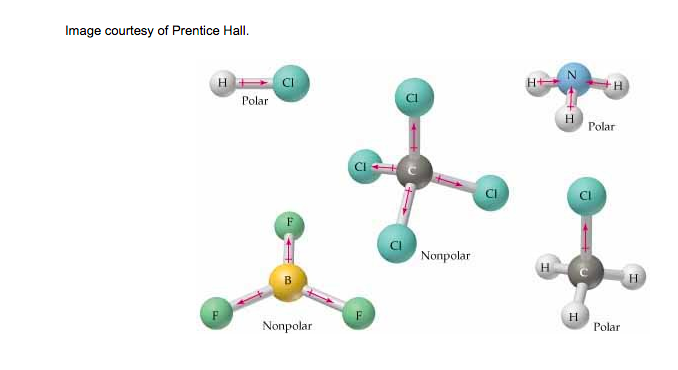
Below are pictures that demonstrate how the electrons are shared between atoms. If you think of it in terms of tug-of-war, there will either be an even pull on the electrons, or an uneven pull on the electrons. An even pull means that the molecule does not orient in any particular direction, which makes it symmetric and considered to be nonpolar. If there is an uneven pull on the electrons, then the molecule will orient in a particular direction (because the unshared pairs of electrons take up more space than shared pairs) and become asymmetric. These molecules are considered to be polar.
Mission Objectives. You should be able to...
1. Explain the difference between polar and nonpolar molecules.
Because covalent bonding involves sharing of electrons, there can be differences in how the electrons are shared between the bonded atoms. The character (or behavior) of the molecules depends on the kind of and number of atoms joined together, which in turn, determine molecular properties.
Below are pictures that demonstrate how the electrons are shared between atoms. If you think of it in terms of tug-of-war, there will either be an even pull on the electrons, or an uneven pull on the electrons. An even pull means that the molecule does not orient in any particular direction, which makes it symmetric and considered to be nonpolar. If there is an uneven pull on the electrons, then the molecule will orient in a particular direction (because the unshared pairs of electrons take up more space than shared pairs) and become asymmetric. These molecules are considered to be polar.
Mission 4: What's in a Name?
Mission Objective. You should be able to:
1. Name covalent compounds according to IUPAC rules.
2. Write covalent formulas.
Mission Objective. You should be able to:
1. Name covalent compounds according to IUPAC rules.
2. Write covalent formulas.
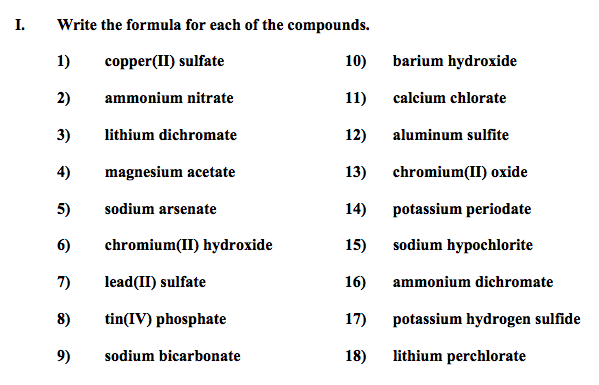
Let's practice!!!