Content Review:
Textbook Readings: Section 1.3
Student Missions:
Mission 1: The Problem is the Solution!
Mission Objectives. You should be able to...
1. Describe the components of a solution
2. Solve problems using molar concentration, amount of solute, and volume of solution.
3. Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard.
Below is an overview of solution chemistry. Terms to know: solute, solvent, molar concentration, dilute, stock solution, volumetric analysis and titration.
Molar concentration of a solution is defined as the amount in moles of a substance dissolved in 1L (1 dm3) of a solvent (usually water). The formula for concentration is on page 31 of your textbook.
Textbook Readings: Section 1.3
Student Missions:
Mission 1: The Problem is the Solution!
Mission Objectives. You should be able to...
1. Describe the components of a solution
2. Solve problems using molar concentration, amount of solute, and volume of solution.
3. Use of the experimental method of titration to calculate the concentration of a solution by reference to a standard.
Below is an overview of solution chemistry. Terms to know: solute, solvent, molar concentration, dilute, stock solution, volumetric analysis and titration.
Molar concentration of a solution is defined as the amount in moles of a substance dissolved in 1L (1 dm3) of a solvent (usually water). The formula for concentration is on page 31 of your textbook.
Solution Concentration
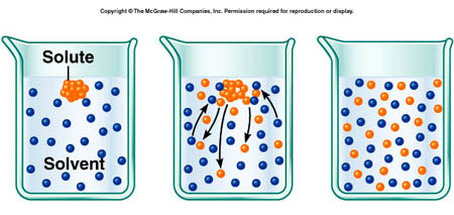
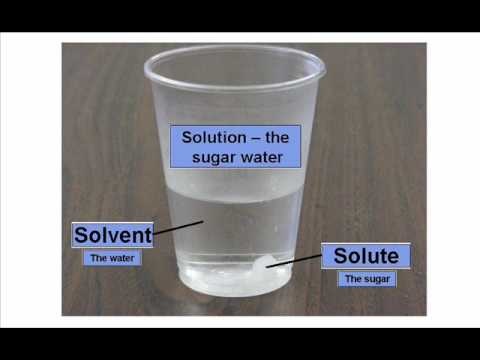
Solutions are homogenous mixtures that exist in all states of matter. Examples include solid/solid solutions, such as brass (copper and zinc) and bronze (copper and tin), liquid/liquid, such as seawater and wine (ethanol and water), and gas/liquid, such as Coca-Cola (carbon dioxide in a sugar syrup). Commonly, we deal with liquid solutions. A solution contains two parts: the solute and the solvent. The solute is the substance that dissolves, and the solvent is the substance that does the dissolving. Water is a universal solvent.
Solution concentration refers to the amount of solute dissolved in a given amount of solvent. A dilute solution contains a small quantity of solute relative to the solvent, whereas a concentrated solution contains a large quantity of solute relative to the solvent. Solutions can be expressed as percent by mass, percent by volume, and molarity. Molarity is the amount of moles of solute per liter of solution. The unit for molarity is either "M" for "molar," or as noted in the IB, "mol dm^-3" Use of the term "molarity" is being replaced with mol dm^-3, and the word itself will not be used in exams. "M" is now being used to express molar mass.
1 dm3 is the same as 1 L.
Concentration of solution (mol dm^-3) = amount of solute in moles/volume of solution in dm^3. Shortcut is to use the following method: c = n/V where "c" is concentration, "n" is the number of moles of solute, and "V" is the volume of the solution in dm^3. To find the moles of solute in a solution, the equation becomes n = cV.
Note: Be careful with distinguishing between "dm^3" and "dm^-3." The three becomes negative when it is no longer in the denominator.
Brackets [ ] are used to represent the concentration of a substance. For example, [HF] represents the concentration of hydrofluoric acid.
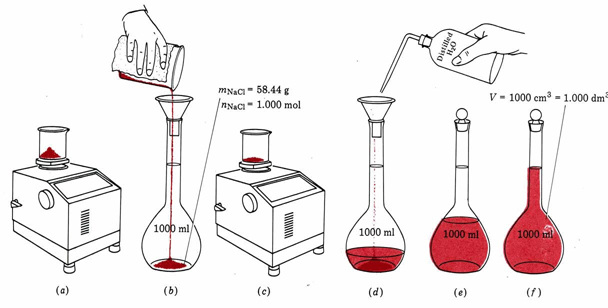
Standard solutions are solutions of known concentration. A specific amount of solute is measured and transferred to a volumetric flask that has been calibrated for a specific volume. The solvent is added steadily using a swirling technique to help in dissolution until the final volume is reached.
Another unit of concentration is ppm, or parts per million. This is one part per 1,000,000 by mass and is normally used in describing very low concentrations in air and water pollution.
The below image shows how a solution is prepared using appropriate equipment.
1 dm3 is the same as 1 L.
Concentration of solution (mol dm^-3) = amount of solute in moles/volume of solution in dm^3. Shortcut is to use the following method: c = n/V where "c" is concentration, "n" is the number of moles of solute, and "V" is the volume of the solution in dm^3. To find the moles of solute in a solution, the equation becomes n = cV.
Note: Be careful with distinguishing between "dm^3" and "dm^-3." The three becomes negative when it is no longer in the denominator.
Brackets [ ] are used to represent the concentration of a substance. For example, [HF] represents the concentration of hydrofluoric acid.
Standard solutions are solutions of known concentration. A specific amount of solute is measured and transferred to a volumetric flask that has been calibrated for a specific volume. The solvent is added steadily using a swirling technique to help in dissolution until the final volume is reached.
Another unit of concentration is ppm, or parts per million. This is one part per 1,000,000 by mass and is normally used in describing very low concentrations in air and water pollution.
The below image shows how a solution is prepared using appropriate equipment.
|
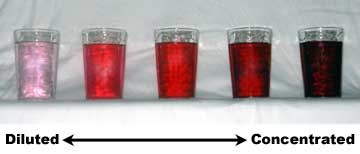
Dilutions. When solutions are diluted, the concentration is reduced. Diluting a stock solution means that more solvent is added. For aqueous solutions, distilled water must be used.See the image to the right. Notice how the color of the solution changes based on how much water is added to the glass. Concentrated solutions have a large amount of solute. Dilute solutions by comparison have a large amount of solvent.
As a solution is diluted, the number of moles (n) of solute remains the same, but as they spread out through the solvent, the concentration (c) decreases. c1V1 = c2V2 (where "c" = concentration, "V" = volume, "1" = initial conditions and "2" = final conditions). This is how to calculate concentration changes on dilution. |
Doc Brown's chemistry page has a nice summary regarding concentration. Remember, however, that the term "molarity" is falling out of use, and instead "c" for concentration should be used in its place. You should go through the practice problems which include the solutions (get it?).
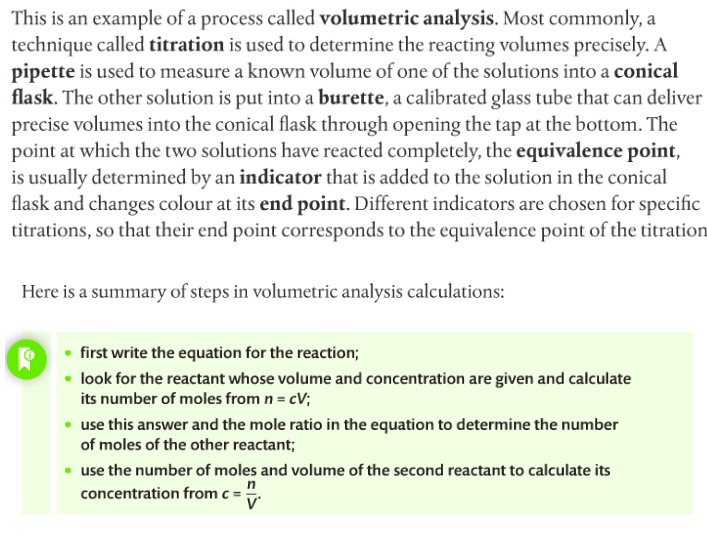
You have to complete a titration practical. Quantitative analysis includes a range of techniques to determine the amount or concentration of an analyte. An analyte is the substance under analysis.
Volumetric analysis is a quantitative technique used by chemists involving two solutions. A titration involves a standard solution of known concentration which is added to a solution of unknown concentration until the reaction is complete. The reaction progress is monitored through color changes using indicators.
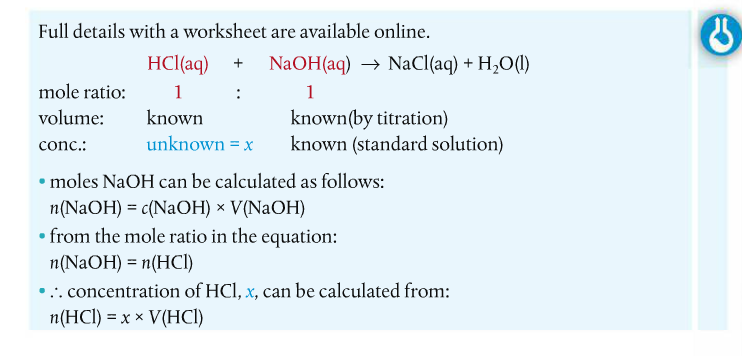
How do you find the concentration of unknown solutions (such as acids)?? If you react the solution with a standard basic (or alkaline) solution and determine the exact volumes that react together, you can determine the unknown concentration of the acid. Here is an example from the Pearson (2014) text.
You have to complete a titration practical. Quantitative analysis includes a range of techniques to determine the amount or concentration of an analyte. An analyte is the substance under analysis.
Volumetric analysis is a quantitative technique used by chemists involving two solutions. A titration involves a standard solution of known concentration which is added to a solution of unknown concentration until the reaction is complete. The reaction progress is monitored through color changes using indicators.
How do you find the concentration of unknown solutions (such as acids)?? If you react the solution with a standard basic (or alkaline) solution and determine the exact volumes that react together, you can determine the unknown concentration of the acid. Here is an example from the Pearson (2014) text.
Homework. Solutions are included so you can check your work.
Concentration Dilution Solution Preparation
Concentration Dilution Solution Preparation